Ionic Compounds
advertisement

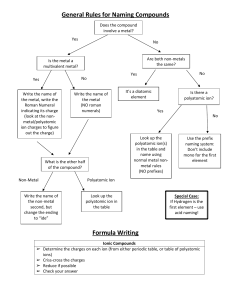
Chemistry – Rules for Naming Compounds Science Ionic Compounds Metals and Non-metals Molecular Compounds Transition metals and Non-metals Polyatomic ions hydroxide nitrate bicarbonate Metal + chlorate carbonate sulfate phosphate Non metals only OHNO3HCO3ClO3CO3-2 SO4-2 PO4-3 ammonium NH4+ Naming Naming 1. Determine the charge of the nonSimple – change the ending metal and record above the element. of the non-metal to “ide”. 2. Determine from the formula, the Do not use captials. charge of the metal required to balance the non-metal. 3. Write this charge in Roman numerals beside the metal in brackets. 4. Change the ending of the non-metal to “ide” (unless it is a polyatomic ion) Formula 1. Determine their ionic charge and write above the element. Formula 1. Write the charge above each element. The charge of the transition metal will be given in its 2. Choose a method such as name. 2. Choose a method such as - Cross- Cross-over method over method - draw the ions - draw the ions to determine the formula to determine the formula. + other NH4+ Naming 1. If the metal comes from group 1 or 2 , use its name with the polyatomic name. OR 2. If the metal comes from the transition metals, apply the rules in column 2 for transition metals. Formula 1. Write the charge above each element. The charge of the polyatomic ion can be found above. The charge of the transition element will be given. 2. Use the cross-over method to determine the formula. You may need to bracket the polyatomic ion if you need more than one of them to balance the charge. Naming Use the following prefixes infront of the element's name. 1 – mono 2 – di 3 – tri 4 – tetra 5 – penta 6 - hexa 7 – hepta 8 – octa 9 – nona 10 - deca Formula Replace the prefix in the name with its corresponding number.