RUBRIC Pre-lab:______/5 Section A: ______/5 Section B:______/5
advertisement
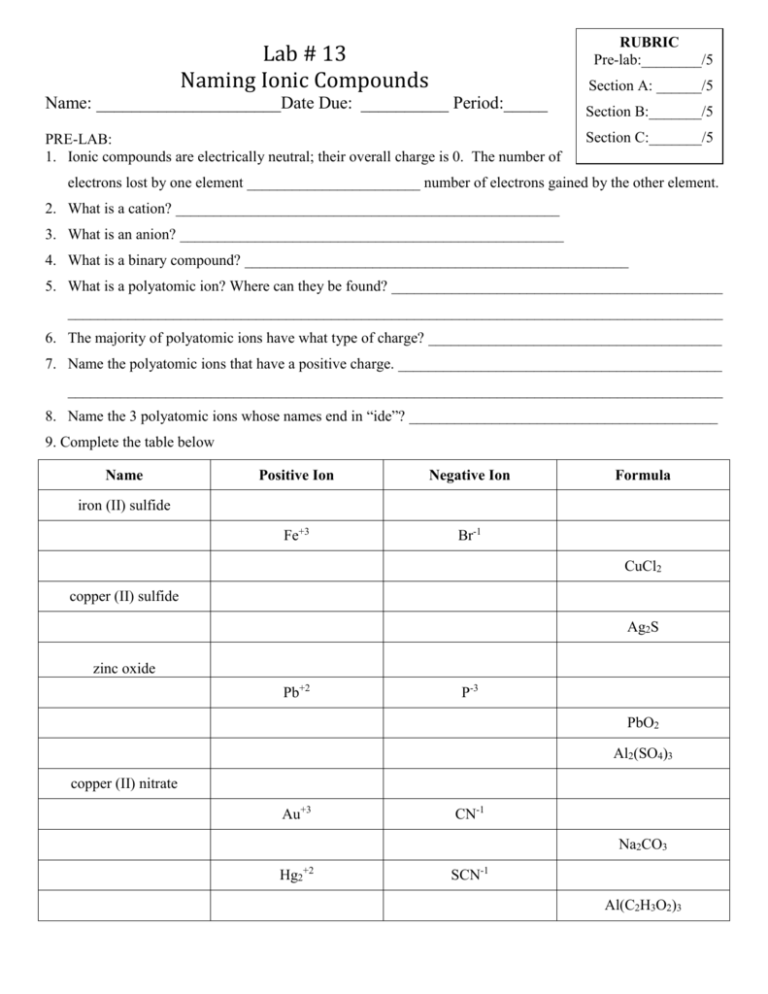
RUBRIC Pre-lab:________/5 Lab # 13 Naming Ionic Compounds Section A: ______/5 Name: _____________________Date Due: __________ Period:_____ PRE-LAB: 1. Ionic compounds are electrically neutral; their overall charge is 0. The number of Section B:_______/5 Section C:_______/5 electrons lost by one element _______________________ number of electrons gained by the other element. 2. What is a cation? ___________________________________________________ 3. What is an anion? ___________________________________________________ 4. What is a binary compound? ___________________________________________________ 5. What is a polyatomic ion? Where can they be found? ____________________________________________ _______________________________________________________________________________________ 6. The majority of polyatomic ions have what type of charge? _______________________________________ 7. Name the polyatomic ions that have a positive charge. ___________________________________________ _______________________________________________________________________________________ 8. Name the 3 polyatomic ions whose names end in “ide”? _________________________________________ 9. Complete the table below Name Positive Ion Negative Ion Fe+3 Br-1 Formula iron (II) sulfide CuCl2 copper (II) sulfide Ag2S zinc oxide Pb+2 P-3 PbO2 Al2(SO4)3 copper (II) nitrate Au+3 CN-1 Na2CO3 Hg2+2 SCN-1 Al(C2H3O2)3 PROCEDURE: 1. Match up the positive and negative ions for the following compounds below. Be sure to determine the number of each ion needed so that the compound formed will be electrically neutral 2. Once completed, get your teachers approval, then write the formula, name of each compound, A) Group 1or 2 or 13 metals + non-metal Metals and Formula Nonmetal Sodium and Bromine Magnesium and Chlorine Aluminum and Sulfur Potassium and Oxygen Your own group 1,2,or 13 metal and nonmetal Teachers approval_________________ Name of Compound Lewis Dot Structure B) Transition metal + non-metal Metals and Formula Nonmetal Iron (+2) and Chlorine Iron (+3) and Chlorine Vanadium (+5) and Oxygen Manganese (+2) and Oxygen Your own transition metal and nonmetal Teachers approval_______________ C) Compound Containing Polyatomic Ion Metals and Formula Nonmetal Magnesium and Nitrate Ammonium and Permanganate Sodium and Carbonate Potassium and Sulfate Your own cmpd containing a polyatomic ion Teachers approval______________ Name of Compound Name of Compound