Ionic Formula Dissection Activity
advertisement

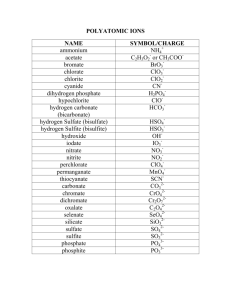
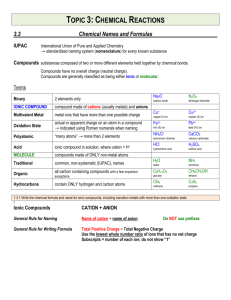
Ionic Formula Dissection Activity Background A chemical formula is a combination of symbols and numerical subscripts that represent the composition of a compound. The symbol indicates which elements are present and the numerical subscripts indicate how many atoms of each element make up the compound. The subscripts can be predicted using ion charges. Ions are atoms that have acquired a charge. Ions can be cations (having a positive charge) or anions (having a negative charge). Ions consisting of more than one atom are called polyatomic ions. It is important that all scientists use the same system for writing chemical formulas. This helps to ensure clear and consistent transmission of information. Use the following rules when writing chemical formulas: Rule 1: Compounds are neutral, so the cation/anion charges must add up to 0. (Na1+ Cl1- NaCl (1+) + (1-) = 0 Rule 2: Subscripts are used to balance ion charges so that the total charge on the compound is 0. Na1+ O2- Na2O 2(1+) + (2-) = 0 Rule 3: If a subscript must be used to balance the charge of a polyatomic ion, the polyatomic ion must be enclosed in parentheses and the subscript put on the outside of the parentheses. Al3+ NO31- Al(NO3)3 (3) + 3(1-) = 0 Purpose In this activity you will “Dissect formulas into cations, anions, and polyatomic ions Investigate the structure of a chemical formula Learn how to name chemical formulas Procedure Use your periodic table to help you ‘dissect’ each chemical formula in the data table into cations, anions, and/or polyatomic ions. Write the symbol and charge of each cation or anion. Then name it. Write the formula for each polyatomic ion. Then name it. Prelab 1. What are the parts of a chemical formula and what do they tell you about the formula? 2. What is the difference between an ion and a polyatomic ion? Give an example of each. 3. Prove Rule #1 and #2 using CaF2. (State each rule and then prove each rule) 4. Count and identify the ions in this formula: (NH4)2 CO3 5. Why are the parentheses necessary in question 4? STOP: PROGRESS CHECK!!!! Data A. B. C. D. E. F. NaCl Na2CO3 NaOH Na2SO4 Na3PO4 NaNO3 1. NH4Br (NH4)2CrO4 (NH4)2S (NH4)2SO3 (NH4)3PO3 NH4C2H3O2 2. KCN K2Cr2O7 K2O K2C2O4 K3AsO4 KClO CaSO4 Ca(ClO4)2 Ca(SCN)2 CaSe Ca(HCO3)2 Ca(NO2)2 3. 4. Part 1: Dissect each ionic formula! Dissect each ionic formula above into the cation and the anion. Name each. Ionic Compound Dissection Number/ Letter Formula Cation/Positive Polyatomic ion Name Anion/Negative Polyatomic ion Name 1A NaCl Na1+ Sodium ion Cl1- Chloride 1B 1C 1D 1E 1F 2A 2B 2C STOP: PROGRESS CHECK!!!! 2D 2E 2F 3A 3B 3C 3D 3E 3F 4A 4B STOP: PROGRESS CHECK!!!! 4C 4D 4E 4F Part 2: Name each chemical formula! The cation name is the element name from the periodic table, or ammonium (a positive polyatomic ion). The anion name ends in -ide or it comes from the polyatomic ion reference sheet. Make no alterations to the polyatomic ion name! EXAMPLE 1: NaCl is the sodium ion and the anion chloride. The name of this formula is sodium chloride. EXAMPLE 2: (NH4)2 CO3. - NH41+ is a polyatomic ion called ammonium and CO32- is a polyatomic ion called carbonate. The formula name is ammonium carbonate. Ionic Compound Nomenclature Number/ Letter Formula Formula Name 1A NaCl Sodium chloride 1B 1C STOP: PROGRESS CHECK!!!! 1D 1E 1F 2A 2B 2C 2D 2E 2F 3A 3B 3C 3D 3E 3F STOP: PROGRESS CHECK!!!! 4A 4B 4C 4D 4E 4F Part 3: Write each chemical formula! 1 1. beryllium hypochlorite ______________________ 2. barium bicarbonate________________________ 3. ammonium dichromate_____________________ 4. strontium sulfide__________________________ 5. lithium selenide__________________________ 6. aluminum carbonate_______________________ 7. cesium oxide_____________________________ 8. calcium bromide__________________________