Polyatomic Ions Reference Sheet: Names, Symbols, Charges
advertisement
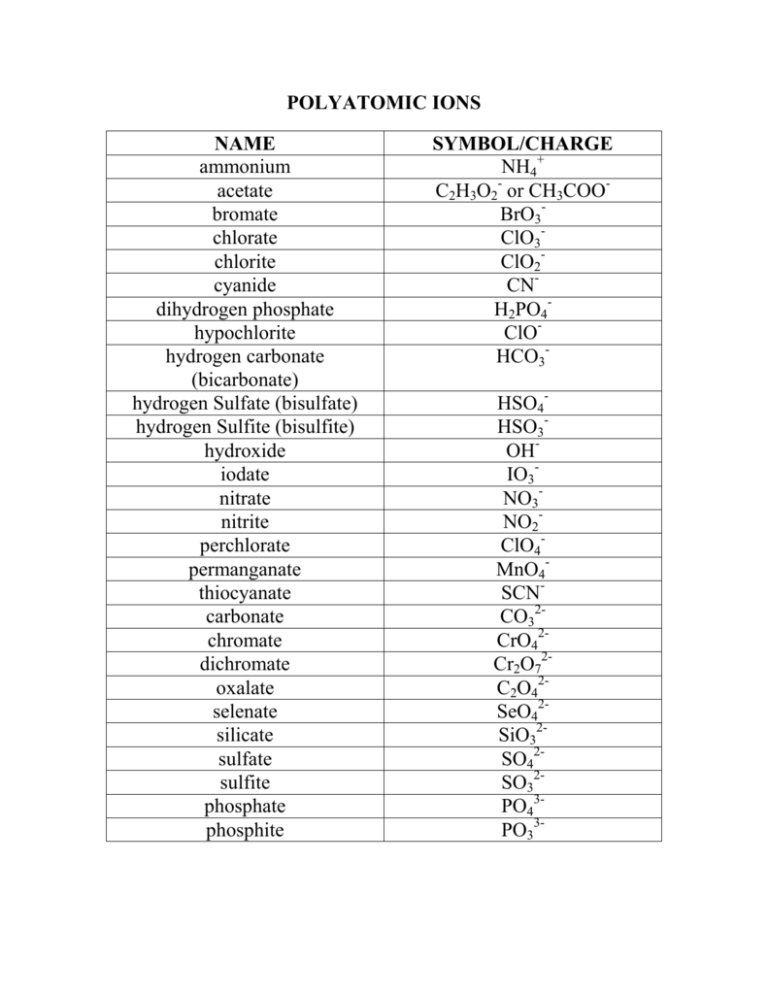
POLYATOMIC IONS NAME ammonium acetate bromate chlorate chlorite cyanide dihydrogen phosphate hypochlorite hydrogen carbonate (bicarbonate) hydrogen Sulfate (bisulfate) hydrogen Sulfite (bisulfite) hydroxide iodate nitrate nitrite perchlorate permanganate thiocyanate carbonate chromate dichromate oxalate selenate silicate sulfate sulfite phosphate phosphite SYMBOL/CHARGE NH4+ C2H3O2- or CH3COOBrO3ClO3ClO2CNH2PO4ClOHCO3HSO4HSO3OHIO3NO3NO2ClO4MnO4SCNCO32CrO42Cr2O72C2O42SeO42SiO32SO42SO32PO43PO33- Rules for Naming and Writing Formulas for Ionic Compounds Containing Polyatomic Ions Polyatomic ions are ions which consist of more than one atom. For example, nitrate ion, NO3-, contains one nitrogen atom and three oxygen atoms. The atoms in a polyatomic ion are usually covalently bonded to one another, and therefore stay together as a single, charged unit. Naming and writing formulas follow the same rules as binary ionic compounds. However, the most important part is recognizing a polyatomic ion is present. How does a person recognize that a polyatomic ion is present? In binary compounds only two different elements are present in the bond. In compounds containing polyatomic ions there are three or more different elements present. Keep in mind that even in compounds containing polyatomic ions, there are still only two ions present (one cation and one anion). Notice that there are a lot more polyatomic anions than cations. Most polyatomic anions, consist of a nonmetallic element combined with different numbers of oxygen atoms (these polyatomic anions are called oxoanions.) Even though it seems there is no simple rule in naming these ions, here are some guidelines to follow: Rule 1. The cation is written first in the name; the anion is written second in the name. Rule 2. When the formula contains two or more of the same polyatomic ion, that ion is written in parentheses with the subscript written outside the parentheses. Note: parentheses and a subscript are not used unless more than one of a polyatomic ion is present in the formula unit (e.g., the formula unit for calcium sulfate is "CaSO4" not "Ca(SO4)"). Rule 3. If the anion is a monatomic ion, the anion is named by adding the suffix ide to the root of the element name (e.g., I- = "iodide"). Note: Greek prefixes are not used to indicate the number of atoms, or polyatomic ions, in the formula unit for the compound (e.g., Ca(NO3)2 is named "calcium nitrate" not "calciuim dinitrate").