Naming and Writing Acids
advertisement

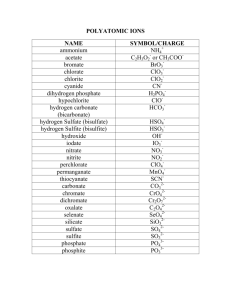
Naming and Writing Acids Going from a formula to a name: Step #1- Ask yourself “is oxygen present?” (example: HCl -- no, H2SO4 –yes) Step #2- If no: Write hydro- then the root of the second element with –ic at the end. Example- HCl is hydrochloric acid If yes: Don’t write hydro, just write the root of the polyatomic ion that has the oxygen. Then, if the polyatomic end with –ite, change it to –ous. If it ends with –ate, change it to –ic. Example- H3PO4 since PO4 is phosphate, the name is phosphoric acid H2SO3 since SO3 is sulfite, the name is sulfurous acid Going from a name to a formula: Step #1 Ask yourself “does the name start with hydro?” Step #2 If yes: a. Write H for hydrogen, then symbol of the second element b. Figure out the oxidation # for each. Hint- Hydrogen will always be 1+ c. Use the criss-cross method to figure how many of each atom you need Example- Hydrosulfuric acid, H1+ and S2- (sulfur) so the formula is H2S If no: a. Write H for hydrogen, then the polyatomic ion the name came from. (If the acid name ends with -ous, the polyatomic will end with –ite, and if the acid name ends with –ic, the polyatomic will end with –ate) b. Figure out the oxidation # for each. This is why you need to memorize the polyatomic ions! c. Use the criss-cross method to figure out how many you need. (Remember to put parenthesis around the polyatomic if you need more than one.) Example: Sulfuric acid comes from sulfate (SO4) which has an oxidation # of 2so the formula is H2(SO4) * Bases contain OH- so if you see that, don’t try to name it like an acid, just use hydroxide as the name.