Polyatomic Ions and Molecular Nomenclature
advertisement

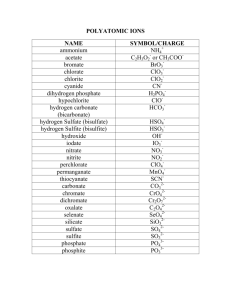
Polyatomic Ions and Molecular Nomenclature Lesson 15 October 25th, 2010 Polyatomic Ions Ions that are composed of one atom are called monatomic ions. Monatomic Ion- an ion composed of only one atom. We have only looked at these so far Polyatomic Ions Polyatomic Ions are ions that are composed of more than one atom. The entire molecule carries a charge to it. Example NO3- SO42- O PO43- 2- O O S O O P O O O 3- Bonding Ionic Bonding with polyatomic ions occurs in the same manner as it does with standard ionic molecules. - Use the crossover method - Be sure that the charge that is crossed over applies to the whole polyatomic ion. If the charge is greater than 1, use brackets around the polyatomic ion to indicate the number applies to the whole ion. Example 1 Step 1 Na + NO3- Step 2 1+ Na Step 3 1- 1+ NO3 Na 1NO3 Step 4 NaNO3 Example 2 Step 1 Mg + NO3- Step 2 2+ Mg 1NO3 Step 3 2+ Mg 1NO3 Step 4 Mg (NO3)2 Practice Mg + ClO3- = Na + SiO32- = Fr + HS- = NH4+ + Cl = Li + Cl = Be + O22- = Sr + PO43- = Al + BO33- = Al + SO42- = Na + OH- = Naming Ionic Polyatomic compounds: IUPAC Multivalent: Metal (charge) polyatomic ion Monovalent: Metal polyatomic ion Tertiary ionic compounds are comprised of a metal ion and a polyatomic ion. Write the polyatomic ions in the same way as monatomic ions. Examples NaOH = Cu(ClO4)2 = sodium hydroxide (NOT MULTIVALENT) copper (II) perchlorate Tin (IV) chlorate = Sn (ClO3)4 Practice Copper (I) hydroxide = Aluminum sulfate = = Cu+ OH- Al3+ SO43- = = AlSO 4 CuOH CrCO3 = Cr2(CO3)3 = Cr2+ CO32- Cr3+ CO32- =Chromium (II) Carbonate Chromium (III) Carbonate Naming Binary Molecular Compounds: IUPAC 1. 2. Write down the name of the first element. If there is more than one atom of this element attach a Greek prefix. (if there is only one atom do not attach the prefix) Attach a Greek prefix (relating to the number of atoms) to the second elements name and add -ide. Prefixes # of atoms 1 2 3 4 5 6 7 8 9 10 Prefix Mono Di Tri Tetra Penta Hexa Hepta Octa Nona Deca Example: CO = Carbon monoxide CO2 = Carbon dioxide Additional Rules Elements that are commonly bonded to themselves in nature do so to make themselves more stable. These molecules are known as diatomic Molecules. Some common examples include (O2, H2, N2, Br2, Cl2, S8). When we name them they retain their element name without the use of prefixes. Example. O2 is not dioxide. It is just Oxygen gas. Practice HF = Hydrogen monofluoride PBr =Phosphorous monobromide carbon tetrafluoride = CF4 Silicon dibromide =SiBr2 Complete all of the practice Questions Unit Test On Thursday