Link to a copy of the exam key, Form B
advertisement
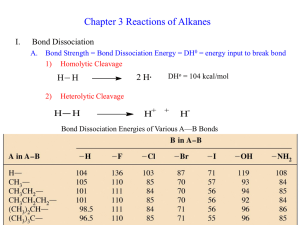
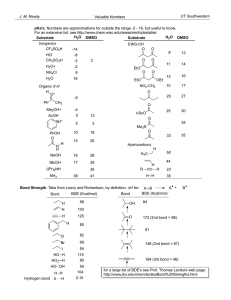
CH 334 First Midterm Exam FORM_B Wednesday, October 17, 2012 Name:____Key____________________________________________ You may use molecular model kits but no other material with chemical information on it. Please do not use MP3 players or other electronic gadgets other than calculators. Useful Information: 1. (15 points) Draw correct bond-line structures (show all lone pairs) for the following compounds. Please include hydrogens and include formal charges where appropriate. a. (CH3)3NO b. C2H6O (any isomer) c. CH3C(H)O 2. (15 points) Draw the major resonance forms for the following molecule. Indicate which atom(s) will be Lewis basic. 4 points per resonance form. The two oxygens will be Lewis basic. (3 points) There is a minor form that places a lone pair at C-2 that illustrates some Lewis basicity for that carbon. 3. (10 points) Label the hybridization of each carbon in the molecule shown below. -2 points for each error/omission up to 10 points. 4. (15 points) The three highest-energy, occupied molecular orbitals of methyleneimine, H2CNH, are sketched in simplified form below. Energies of the MO are included in eV. Lone pair π-bonding σ-bonding a. Label each MO above as either (mostly) lone-pair, sigma-bonding or pibonding. See above. -2 for each error (max -5) b. This molecule reacts with strong Bronsted acids as a proton acceptor. Draw the structure (and any important additional resonance structures) for the protonated product that forms. Be sure to clearly express the bond angle the new proton forms with other atoms. All atoms are coplanar; the H-N-C angles and the H-N-H angles are close to 120°. 2 points for each resonance structure; 1 pt. for bond angle c. Explain why protonation occurs where you show it, based on the MO description provided above. The most reactive electrons are the lone pair. The resulting cation is resonance stabilized so long as all hydrogens are coplanar with C and N. 5. (15 points) Consider the alkane 2,3-dimethylhexane. a. Draw this molecule in bond-line notation. 5 points b. Draw a Newman projection of the most stable arrangement around the C2C3 bond. (You may abbreviate any longer chain using its formula.) 5 points c. How many gauche interactions are there? Circle them on your diagram. 2; circled above. Note that any other rotational isomer will have 3 gauche interactions. 5 points. -2 for each incorrect interaction circled or each one missed. 6. (30 points) Compound B below (shown in bond-line notation) has 5 distinct C-H bonds. a. Draw in one of each distinct kind, and label each one as primary, secondary or tertiary. 5 points b. Compound B reacts with Br2 (using light to initiate the reaction) to form a single major product. Draw that product. 5 points c. Using bond dissociation energies from the table on the last page, calculate the net ΔH° for the reaction you drew in (b). Break Br-Br: Break 3° C-H: +46 kcal/mol +96.5 kcal/mol Form H-Br: Form 3° C-Br: -87 kcal/mol -71 kcal/mol Net ΔH°r -15.5 kcal/mol (You may also properly split this into the separate reaction mechanism steps; the H-atom transfer to Br has a net ΔH°r of +9.5 kcal/mol, and the Br atom transfer has a net ΔH°r of -25.0 kcal/mol. The sum of the two is still -15.5 kcal/mol.) 10 points d. We photochemically cleave Br2 to form two Br atoms as the initiation step of the reaction. Show, using the correct curved arrows, the two steps involved in radical chain propagation that gives the product you claim is formed in (b). 10 points; 5 points each reaction Bond strengths (kcal/mol): F-F Cl-Cl Br-Br I-I H-F H-Cl H-Br H-I CH3-H CH3CH2-H (CH3)2CH-H (CH3)3C-H CH3-F CH3-Cl CH3-Br CH3-I CH3CH2-F CH3CH2-Cl CH3CH2-Br CH3CH2-I (CH3)2CH-F (CH3)2CH-Cl (CH3)2CH-Br (CH3)2CH-I (CH3)3C-F (CH3)3C-Cl (CH3)3C-Br (CH3)3C-I 38 58 46 36 136 103 87 71 105 101 98.5 96.5 110 85 70 57 111 84 70 56 111 84 71 56 110 85 71 55