Molecular structure: how are the different atoms bonded together
advertisement

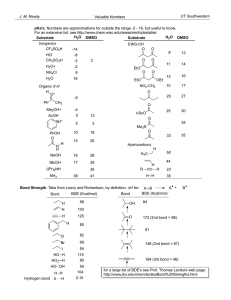
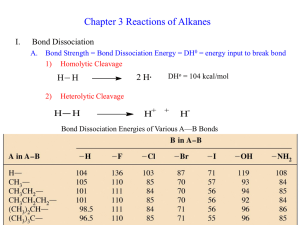
Molecular structure: how are the different atoms bonded together (topology) and what is the 3-dimensional configuration (geometry)? Lewis Theory: Each atom contributes its valence electrons. A covalent bond is formed of 2 e-’s and every atom tries to satisfy the octet rule while attaining a formal charge of zero. The 3-dimensional structure is determined by maximizing symmetry and minimizing contacts. H C 1 e- N 4 e- X P S 7 e- 5 e- 6 e- 3-5 bonds 2-4 bonds O 6 e- 5 e- 1 bond 4 bonds 3 bonds 2 bonds 1 bond Formal Charge = #valence e- - (#bonds + lone-pair e-) O N C O N water H2O, 8 e- H O H tetrahedral, – ~ 109˚ O H O CH2O, 12 e- H O C H H nitrate ion C C H H H H H planar, – ~ 120˚ C H H - O N O H C - O trigonal planar, – ~ 120˚ C H C2H4, 12 e- NO3-, 24 e- C C H formaldehyde ethylene O N O O N O O O - O ??? N O - O O 1 H n-butane H C4H10, 26 e- H H H H C C C C H H H H H H H H C C H C H H H iso-butane, C4H10 H3C CH H H H H H CH3 C H CH3 H C H C H C C H H H H3C CH3 CH3 H H 1-butene, C4H8 H2C C H CH2 CH3 H H H3C C H C H CH3 CH3 H3C H H H H3C H H H C H H 2-butene, C4H8 H C CH3 cis trans Review of Molecular Orbital Theory Hydrogen atom proton (+) charge Electron (-) charge. NOT a “cloud” of negative charge but a particle w/ wave-like properties. Its location at any point in time is unknown but there is a 99% probability that at any time it can be found within some volume, which is defined as the “orbital”. For any given atom or molecule, there are discrete orbitals defined by energy and shape . . . y3 E y2 The orbital for a molecule is simply the linear combination of the atomic orbitals for all the atoms involved. For example, to make the H2 molecule: y1 -ground state orbital energy diagram for a neutral hydrogen atom. The lowest energy orbital for hydrogen is an s orbital ± y1 = ?? y1 2 Making of Molecular Orbitals: Hydrogen Molecule ± y1 s* orbital is anti-bonding, which means that it has no e- density between the two atoms- i.e., they’re not bonded y1 Anti-bonding s* E y1 y1 s Bonding 2 e-/orbital! s orbital is defined by the maximum e- density between the atoms along the internuclear axis Making of Molecular Orbitals: p Bonds p* orbital is anti-bonding, which means that it has no e- density between the two atoms- i.e., they’re not bonded ± y1 y1 Anti-bonding p* E y1 y1 p Bonding p orbital is defined by the maximum e- density between the atoms ABOVE the internuclear axis- none along axis! 3 Orbital Hybridization: Methane Methane = CH4 •Carbon = 6 protons, 6 electrons •Configuration = 1s22s22p2 (px, py, pz) •4 Hydrogen atoms = 4 electrons (s) 4 valence e- in an s and 3 p orbitals Have 8 bonding electrons and with 2e- per bond, need 4 bond orbitals x y H z Hybridize (s + 3 p) x C H H y H + 4 H atoms z tetradedral geometry An sp3 hybridized carbon atom x y z 4 sp3 orbitals Making More Complex Molecules Rotational barrier is about 85 kcal/mol Lewis Formula H H C H H C H C C H C C One less bond than normal: (-) charge Formamide, HCONH2 Rotational barrier is about 15 kcal/mol ... N H H H Resonance Forms N+ H H N C H .. .. .. H C -O .. . .. O - O H H sp2 carbons H H C H H p bond H Ethylene, C2H4 H One more bond than normal: (+) charge 4 A Momentary Digression …. Thermodynamic Considerations ….. Kinetic Considerations…… Consider A + B = A-B, Keq=[A-B]/[A][B] And Free energy, DG = -RTlnKeq R = ideal gas constant = 2 cal/mol Kelvin Let’s say that the concentration of product, [AB], is 10-fold higher than reactants at equilibrium (favorable). Rate a e-Ea/RT where Ea is the activation energy (barrier) Solve for DG and get DG = -1.4 kcal/mol At room temperature (T = 300 K), RT = 600 cal/mol = 0.6 kcal/mol That is, each factor of 10 (10-fold) is worth 1.4 kcal/mol. The average energy is RT- many have more, ie 5-10RT or 3-5 kcal/mol At room temp. ~1% of the molecules have ~3 kcal/mol thermal energy H H H H H C H H C H H Rotational barrier is about 1.5 kcal/mol- spins like a top H H H H C C H H H C C H H H H H H H H CH3 H H H H C H H H C H H CH3 C H C C H H H C Barrier here is about 5 kcal/molspins like a sputtering top Rotational barrier is 85 kcal/mol Does not happen…. H H C C H H H H H This conformation or rotamer is about 1 kcal/mol higher in energy- ratio ~ 1-to-7 H CH3 H CH3 5