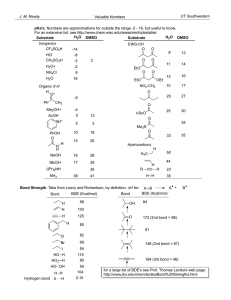
Chapter 3 Reactions of Alkanes 2 H H
advertisement

Chapter 3 Reactions of Alkanes I. Bond Dissociation A. Bond Strength = Bond Dissociation Energy = DH0 = energy input to break bond 1) Homolytic Cleavage H H 2) 2H DHo = 104 kcal/mol Heterolytic Cleavage H H H+ + H- Bond Dissociation Energies of Various A—B Bonds 3) H H H OH H+ OH + OH + OH- DHo = 119 kcal/mol DE much smaller Polar solvents stabilize ions 4) B. DHo depends on how well orbitals overlap: HF > HCl > HBr > HI Stability of Radicals 1) Alkyl Radicals: 3o > 2o > 1o > Methyl Stability CH3-H 105 CH3CH2-H 101 DHo (CH3)2CH-H 98.5 (CH3)3C-H 2) 96.5 Hyperconjugation Explains Radical Stability a) Hyperconjugation = delocalization of s-bond electron pair into a partly empty p-orbital Delocalization = spreading e- over multiple atoms b) c) d) Methyl Radical Alkyl Radicals are planar (sp2) with single e- in p-orbital Must have s-bond in proper geometry to help Hyperconjugation is similar to p-bonding, but has odd # of e- C. Pyrolysis 1) Pyrolysis = breaking of CH, C-C bonds with heat D CH3 + Radical Combinations + + 2) II. Cracking = breaking into smaller fragments a) Control with catalysts b) Petroleum into gasoline Radical Chain Mechanisms A. Chlorination of Methane CH4 + 1) 2) Cl2 Dhn CH3Cl + HCl DE = -25 kcal/mol D = heat, hn = light Ea is high, so energy is required to start the reaction B. Mechanism A. Initiation B. Propagation 1) Cl 2) C. Cl + Dhn Cl HCl + H-CH3 CH3 + Cl2 Termination CH3 CH3Cl + Cl Cl2 2 Cl Cl + 2 CH3 1) 2) 2 Cl CH3 CH3Cl CH3CH3 Termination is rare because radicals are few, small odds of meeting Radical chains use up “fuel” before termination C. Reactivities of other Halogens 1) F2 > Cl2 > Br2 > I2 2) Propagation 1 a) F2 DH = -31 kcal/mol b) Cl2 DH = +2 kcal/mol c) Br2 DH = +18 kcal/mol d) I2 DH = +34 kcal/mol 3) Hammond Postulate a) Early TS = little bond breaking = exothermic reaction b) Late TS = much bond breaking = endothermic reaction c) At TS, Bond Breaking Energy = Bond Forming Energy 4) Propagation 2 a) Exothermic for all halides b) Total DE for Prop. 1 and Prop. 2 F(-) > Cl(-) > Br(-) > I(+) Iodine won’t react with methane to give MeI