notes 10-6-2006
advertisement

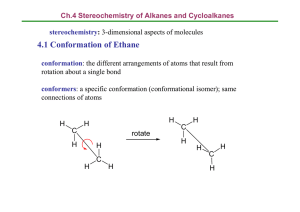
10/06/2006 Conformational, Steric and Stereoelectronic Effects (C&S A, Chapter 3, p. 117-178); Handouts 18 and 19 Conformation of acyclic molecules Total energy related to geometry- can be divided up into specific structural features Recognizable connections between energy and geometry: non-bonded repulsions, ring strain in cyclic systems, tortional strain from non-optimum rotational alignment, destabilization from distortions of bond lengths and bond angles. There are also stabilizing interactions that result from geometrical constraints. • Most important are stereoelectronic effects i.e., a particular geometrical relationship needed for most stabilizing interaction - geometry dependent orbital interactions. • Consider also H-bonding and dipolar interactions When a bond can be rotated, it happens and the molecule exists in the lowest energy form as determined by the rotation of various bondsThe various shapes the molecule takes with certain energy minima are called conformations. - Descriptions can be approached by classical-mechanical or from an MO view point -Strain - caused by non-ideal geometry Contribution to steric energy and molecular mechanics (read C&S A, section 3.1, p. 124) Non-bonded interactions difficult to estimate- Could be attractive or repulsive. • Energy as a function of internuclear distance Morse potential (see CSA fig. 3. 2, p. 126). 10/06/2006 • London dispersion forces • van der Waals radii -van de Waals repulsion- When two atoms (groups) are closer in distance than the sum of their van der Waals radii. H 1.2, F 1.47; CH3 2.0; N 1.55; Cl 1.75; Br P 1.85; 1.80; O I 1.98. 1.52; S. 1.80; Barrier to rotation in ethane (CSA p. 127, Fig. 3.1) -overhead 3 maxima and 3 minima in energy vs dihedral angle. Staggered-minimum Eclipsed -maximum at 2.9 kcal/mol or 12.1 kjoules/mol (1cal = 4.184 Joules) Origin of barrier - unknown - sigma bonds involved are cylindrical- Is it steric?- H’s passing one over the other? Probably not. H too small MO calculations predict correct value Known as Pitzer or tortional strain (Pitzer, R. M. Acc. Chem. Res. 1983, 16, 207) -Barrier due to overlap (exchange) repulsions between the electron pairs in the coplanar C–H bonds. (Other explanation; Coulombic repulsion) Pitzer Strain or Torsional Strain: each C-H eclipsing interaction is worth ~ 1.0 kcal/mol Propane 10/06/2006 2-methylpropane Barrier to rotation in butane (C&S A, p. 121, Fig 3.3) - overhead Resembles ethane - 3 minima, one deeper than the other two Minima are staggered conformations; Maxima - eclipsed conformations Staggered anti - lowest energy - other energies measured from here. Gauche 0. 8 kcal/mol above anti staggered 10/06/2006 Me-Me eclipsed 6.1 Kcal/mol above staggered anti Me-H eclipsed 3.4 kcal/mol above anti-staggered • These are superimposed van der Waals and tortional (Pitzer) strains. Calculate van der Waals contribution in the eclipsed conformations- For Me-Me eclipsed: 6.1(total) - 2.9 (due to Pitzer or tortional strain alone) = 3.2 kcal/mol For Me-H eclipsed: 3.4 - 2.9 = 0.5 kcal/mol; but now there are two such interactions . Therefore the van der Waals contribution per Me-H interaction is 0.5/2 = 0.25 kcal/mol. Population of conformations are related to their energy G0 = -RTlnK = -3.9 kcal/mol (work through the math) Calculate Kequilibrium = (anti/gauche ) =1.9 i.e, 66 % anti and 34 5 gauche Study Table 3.2 calculate % more stable isomer as a functional of G0 Free energy(G0 ) K % more stable isomer -0.000 1 50 -0.502 2.33 70 - 1.302 -2.722 9.00 90 99.00 (practice calculations) In general Staggered minima Eclipsed maxima (ethane and butane) 99 10/06/2006 Staggered anti need not be the minimum. e.g. sometimes attractive forces can prevail - 1-chloropropane gauche more stable Me-Cl attraction! - by H 0.3 ± 0.3 kcal/mol • Total strain can be calculated (molecular mechanics) and there is good correlation between intramolecular strain and bond dissociation energies (See table 3.3) Rotational barriers of groups (Table 3.4) Me - Me 2.9 kcal/mol Me - Et 3.4 (increase of 0.5 kcal/mol per methyl group eclipsing) Me - CH (Me)2 3.9; calculated: 2.9 + 0.5 + 0.5 = 3.9 Me - C( Me)3 4.7; calculated. 2.9 + 0.5 + 0.5 + 0.5 = 4.4 Me - SiH3 1.7 (longer C-Si bond, decreased electron-electron repulsion in eclipsed.) Si - C = 1.87 Å ; C - C = 1.54 Å ethane --> methylamine ----> methanol 2.9 1.98 1.07 (decrease with decreasing number of C–H/X–H eclipsing interactions) CH3–CH2–CH3 (3.4) vs CH3–CH3 (2.8) difference ~ 0.6 Å CH3–NH–CH3 (3.62) vs CH3–NH2 (1.98) difference ~ 1.6 Å CH3–O–CH3 (2.70) vs CH3–OH (1.07) difference ~ 1.6 Å (greater difference in the amine and ether because of shorter C–O and C–N bond distances) Terminal Alkenes 10/06/2006 Carbonyl compounds 1,3-Dienes 10/06/2006 Unsaturated carbonyl compounds Stereoelectronic factors favor coplanar arrangement Aldehydes Ketones Esters