9.10
advertisement

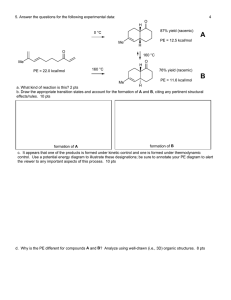
9.10 Use equation 9.31 to find the higher temperature that will allow a decrease in MW by a factor of 100/143 = xn2/xn1. use R = 1.987 * 10-3 kcal/mol-K Ep = 7 kcal/mol, Ed = 27 kcal/mol, and Et = 3 kcal/mol T1 = 65 oC = 338.15 K New temperature to get xn = 100,000 is 75.5 oC (or 348.6 K) The increase in speed of the reaction can be determined by equation 10.30, using rp (T2) / rp (T1) = 45 min / X min (rate = quantity per time, so, rp (T1) = q/45 min, and rp (T2) would produce the same quantity of polymer (q) in less time rp(T2) = q/X min. The same reaction could be done in 19.3 minutes at 75.5 oC if a smaller Mn (100,000) were acceptable. Or, another correct way of saying it, would be that the rate of polymerization could increase by 233 %.