VI-Aldehydes and Ketones
advertisement

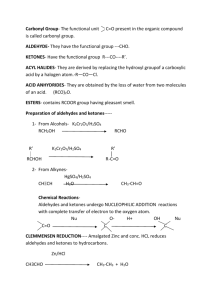
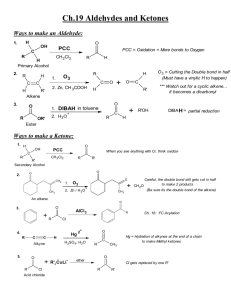
Lecture (9) VI-Aldehydes and Ketones 1-Introduction: Aldehydes are compounds of the general formula R─CHO; ketones are compounds with the general formula R─CO─R' the groups R and R' may be aliphatic or aromatic. Aliphatic aldehydes and ketones have the general formula CnH2nO. Both aldehydes and ketones contain the carbonyl group C=O, and are often referred to as carbonyl compounds. It is the carbonyl group that largely determines the chemistry of aldehydes and ketones. The carbonyl group consists of a sigma bond (σ) and a (П) bond. The carbonyl group is polar; the oxygen has two pairs of unshared electrons. 2-Nomenclature of aldehydes and ketones: (A)-Common name: I-Aldehydes (names derived from acids) Simple aldehydes are usually named after the acids produced by them on oxidation. The ending(ic) of the name of the acid is replaced by the word aldehyde. Examples: HCOOH (formic acid) HCHO (formaldehyde) CH3COOH (acetic acid) CH3CHO (acetaldehyde) C2H5COOH (Propionic acid) C2H5CHO (propionaldehyde C3H7COOH (Butyric acid) C3H7CHO (Butyraldehyde) II-Ketones: The simple Ketones are named by a functional group name. The alkyl groups attached to the carbonyl group are named, and then the word ketone is added. Examples: CH3COC2H5 Ethyl methyl ketone (MEK) CH3COCH3 Dimethyl ketone (Acetone) (B)-IUPAC name: In the IUPAC system, the name of an Aldehyde is drived from the name of the parent alkane by changing the final (-e) to (-al). No numbering is needed as the CHO group always on carbon (1). The name of ketone named by changing the (-e) of the alkane name to (one). Numbering is used when necessary. 51 Examples: ● CH3CHO ●CH3CH=CHCHO ● CH3COCH2CH2CH3 ●CH3COCH=CHCH3 Ethanal 2-butenal 2-Pentanone 3-Penten-2-one 3-Preparation of aldehydes and ketones: (A)-Oxidation of alcohols: K2Cr2O7/H2SO4 RCH2OH RCHO (1°alcohol) aldehyde K2Cr2O7/H2SO4 R1R2CHOH R1COR2 (2°alcochol) ketone (B)-Hydration of alkynes: H2SO4/H2O (1)-HC≡CH Acetylene HgSO4 H─ C = C ─H H OH Vinyl Alcohol CH3CHO acetaldehyde H2SO4/H2O (2)-H3C─C≡CH Propyne HgSO4 (C)-Ozonolysis of alkenes: O3 (1)-H2C=CH2 Ethylene Zn/H3O+ CH3─C=CH2 OH Propenyl Alcohol CH3COCH3 acetone 2HCHO formaldehyde O3 (2)- (CH3)2C=C(CH3)2 2,3-dimethyl-2-butene + Zn/H3O (CH3)2CO acetone 4-Physical Properties: (A)-The polar carbonyl group makes aldehydes and ketones polar compounds, and hence they have higher boiling point than non polar compounds of compared molecular weight. By themselves, they are not capable of intermolecular hydrogen bonding since they contain hydrogen bonded only to carbon, as a result they have lower boiling points compared to alcohols or carboxylic acids. 52 Example: Propionic acid (141°C B.P)>n-butyl alcohol (118°C B.P)> ethyl methyl ketone (80°C B.P)>n- butyraldehyde (76°C B.P)>n-Pentane (36°C B.P) (B)-The lower aldehydes and ketones are appreciably soluble in water, because of hydrogen bonding between solute and solvent molecules. Border line Solubility is reached at about five carbons after that the solubility in water decreases with increasing their molecular weights. (C)-Aldehydes and ketones are soluble in the usual organic solvents like ether and alcohols. 5-Chemical reactions: Addition reactions of aldehydes and ketones: ●The carbon-oxygen double bond is polar and may be attacked either by nucleophile or electrophile. ●The carbonyl group is stabilized by adjacent alkyl groups. Which are electron releasing. Ketone with two R groups more stable than an aldehyde with only one R. Formaldehyde with no alkyl groups is the most reactive of the aldehydes and ketones. RCOR RCHO HCHO Ketone aldehyde formaldehyde Increasing reactivity (A)-Reaction with alcohols: ●The product of addition of one molecule of an alcohol to an aldehyde called Hemiacetal, while the product of addition of two molecules of alcohol is called Acetal (with loss of H2O). ●HemiKetal and Ketal are the responding terms used for ketone products. ●all these reactions are catalyzed by a trace of strong acid. R'OH, H+ RCHO OR' R─ C ─H R'OH, H+ OH Aldehyde OR' R─C─H + H2O OR' hemiacetal (OH+OR on C) 53 acetal (two OR on C) e.g.: CH3COH 2CH3CH2OH, H+ OCH2CH3 CH3 C H +H2O OCH2CH3 acetal Acetaldehyde (B)-Reaction with Grignard reagent: The reaction of formaldehyde with grignard reagent yields a primary alcohol. Other aldehydes yield secondary alcohols. Ketones yield tertiary alcohols. H2O, H+ RMgX + HCHO RCH2OH + MgXOH Formaldehyde (1°alcohol) + H2O, H RMgX + R'CHO R'RCHOH + MgXOH (2°alcohol) + H2O, H RMgX+R'COR'' RR'R"COH + MgXOH (3°alcohol) (C)-Oxidation of aldehydes and ketones: K2Cr2O7/H2SO4 RCHO Aldehyde RCOOH carboxylic acid 54