Aldehydes and Ketones note
advertisement

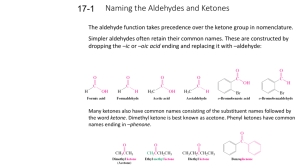
Aldehydes and Ketones: These differ only in that the C=O is terminal on the aldehyde and not on the ketone. Naming the aldehyde, replace the 'ane' of the alkane with 'al' . O H C H Formaldehyde or methanal is a well known biological preservative, "eau de shark" The shortest chain ketone is acetone, know in IUPAC as propanone H H H C H C C H O H ALDEHYDES AND KETONES: These are organic compounds that contain a carbonyl group. It the group is terminal, the compound is classified as an aldehyde; otherwise it is a ketone. Naming an aldehyde: 1. Name the parent alkane. Always give the carbon atom of the carbonyl group the position number 1. 2. Replace the –e at the end of the alkane name with –al. Because the carbonyl group is always given position number 1, you do not need to include a position number for the carbonyl group. 3. When the CHO is attached to a cyclic hydrocarbon, the CHO is given the name -carbaldehyde. Below is cyclohexane carbaldehyde and the carbon with the CHO is numbered 1 in the cyclo chain Naming a ketone: 1. Name the parent alkane. Note that the main chain must contain the C=O group. 2. Replace the –e suffix with -one 3. If more than one ketone group is present, keep the –e suffix and add – dione, -trione, etc. 4. For carbon chains larger than 4, a position number is needed for the carbonyl group. Indicate the position of the C=O as you did the position of a double bond in and alkene and the OH in an alcohol. The carbon where the =O is must be closest to the 1 end of the ketone. H H CH3 H C C C C CH3 H H H O 3-methyl pentan-2-one is *** if an aldehyde and ketone are present – treat the =O of the ketone as an –oxy functional group and the Aldehyde as the parent. Properties of Aldehyde and Ketones: The C=O bond makes aldehydes and ketones polar molecules. There is no hydrogen bonding with these molecules as there is no –OH group. They can however accept hydrogen bonding from water. But there are dipole-dipole attraction between the polar C=O bonds. So, molecules of aldehydes or ketones attract each other more strongly than alkanes with the same molar mass, but less than alcohols of the same size. In general the odour of aldehydes is strong and pungent, while that of ketones is sweet. But higher molecular mass aldehydes have pleasant smells and are often used in perfumes. Reactions of aldehydes and ketones. Alcohols can be reacted in the presence of an oxidizing agent (Cr2O72- + H2SO4) to form an aldehyde or a ketone, depending upon whether the alcohol is primary or secondary. example #1 1 alcohol aldehyde example #2 2o alcohol ketone When you oxidize with H2SO4 as a catalyst, the reaction can be thought of as adding O and removing H2O . This gives a net elimination of H2 Alcohols will also react with acidic Cr2O72- to produce Cr3+ and an aldehyde. (the first Breathalyzer) HW: Physical property summary Guide There are three types of intermolecular attractive forces that help explain the physical properties like relative melting point, boiling point and solubility of organic compounds: Name Origin Non-uniform motion 1. London of electrons (dispersion)forces in atoms and molecules 2. Dipole-dipole interactions 3. Hydrogen bonding Electrostatic attraction between (+) and (-) ends of molecules An especially strong sort of dipole-dipole interaction Type of compounds Result on physical properties Alkanes/alkenes/Alkynes Low PB/BP. More atoms = stronger force = higher BP/MP all molecules having polar covalent bonds or POLAR shapes Raises MP/BP Makes soluble in water More polar = more soluble = higher BP?MP Aldehydes/Ketones ...molecules having H atom(s) covalently bound to N,O, or F Alcohols, NH2, COOH have it Aldehydes/ketones DO NOT Raises Solubility and MP/BP