Other Reactions of Ketones and Aldehydes
advertisement

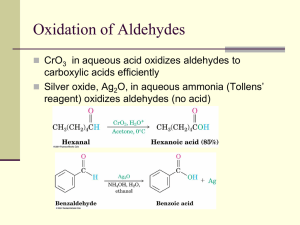
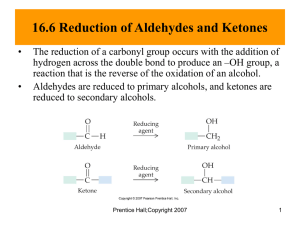
Other Reactions of Ketones and Aldehydes Relative Reactivity of Carboxylic Acid Derivatives Relative Reactivity of Carbonyl-containing Compounds Formation of hydrates (gem-diols) from aldehydes and ketones Mechanism of acid-catalyzed hydration of a ketone Formation of Acetals and Ketals Formation of hemiketals and ketals from ketones The equilibrium can be driven to the right by removal of water from the reaction (through a Dean-Stark trap, or addition of a drying agent) Hemiacetal Full ketals and acetals are quite stable (including stability to strong base), but hemiketals and hemiacetals are in equilibrium with the aldehyde and ketone, with the C=O group usually favored, except in the case of cyclic hemiacetals, like carbohydrates, which exist primarily as cyclic hemiacetals as shown above. Note that the sixmembered ring size is favored. The equilibrium between the open and closed (glucopyranose) form of glucose results in epimerization of the anomeric carbon (the aldehyde carbonyl carbon in the open chain form) Notice that there are two C=O’s, but only the aldehyde (and not the carbamate) carbonyl is affected by these reaction conditions. Note the use of a diol to facilitate transformation to the FULL acetal, through formation of a five-membered ring. Note that only the ketone carbonyl is converted to the ketal (since ketones are more reactive than amides). This (ketalization) reaction is reversible under acidic conditions (and upon the addition of water) Use (of ketal) to ‘protect’ the ketone during reduction of the esters Note that the ketal is formed in step 1, the reduction is performed in step 2, and the ketal is converted back to the ketone in step 3, above. Formation of Oximes and Hydrazones from Aldehydes and Ketones Compared with other imines (C=N), oximes and hydrazones are more stable, due to resonance from the adjacent heteroatom through the C=N. Mechanism of oxime formation Hydroxylamine (usually added as hydroxylamine hydrochloride) Oxime (frequently solid) Use of hydrazine (H2N-NH2) leads to formation of the corresponding hydrazone. Formation of Imines Imines form rapidly from aldehydes and primary amines, but are not stable to hydrolysis (back to the aldehyde and amine), and are rarely isolable. Combination of Imine Formation with Hydride (NaBH3CN) Reduction of Intermediate Imine, to Produce Amine (Reductive Amination) Mechanism of Reductive Amination Procedure Notice that it is the N-protonated iminium ion which is reduced by the hydride reagent. To do this requires a hydride reagent which is stable under slightly acidic conditions. The two most commonly used reagents are: sodium cyanoborohydride, NaBH3CN, and sodium triacetoxyborohydride, NaBH(OAc) 3