In Nu- Addition reactions…
advertisement

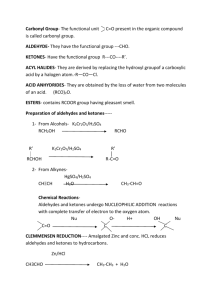
Ch.19 Aldehydes and Ketones Ways to make an Aldehyde: H 1. O OH PCC C R CH 2Cl 2 H Primary Alcohol 2. R H C PCC = Oxidation = More bonds to Oxygen R R O3 1. H C C H 2. Zn, CH 3COOH R' + O O H H O 3 = Cutting the Double bond in half (Must have a vinylic H to happen) R' *** Watch out for a cyclic alkene... it becomes a dicarbonyl C Alkene 3. O O 1. DIBAH in toluene R 2. H3O OR' + C + R R'OH DIBA H = partial reduction H Ester Ways to make a Ketone: 1. H O OH PCC C R R' Secondary Alcohol When you see anything with Cr, think oxidize CH 2Cl 2 R R' O O 2. CH2 1. O O3 2. Zn / H 3O CH3 + + CH3 Careful, the double bond still gets cut in half to mak e 2 products CH 2O (Be sure its the double bond of the alk ene) An alkene O O 3. +R AlCl 3 C C Hg H O R + Cl Acid chloride 2 + H2SO 4, H2O Alkyne 5. Ch. 16: FC Acylation Cl 4. R R - O C R CH3 Hg = Hydration of alk ynes at the end of a chain to mak e Methyl k etones O + R'2CuLi ether Cl gets replaced by one R' R R' Oxidizing Aldehydes: O 1. OH R R H O CrO3 OH H3O Cr = More bonds with Oxygen + R H OH hydrate O O 2. Ag 2O H OH Tollens reagent + NH 4OH, H 2O, ethanol Ag Oxidizing Ketones: 1. O 1. Hot KMnO4 H2O, NaOH 2. H3O COOH Works for symmetrical ketones + COOH Nu-Addition Reactions of Aldehydes & Ketones: O O R OH - H - Nu R R Nu R' Ketone or Aldehyde + R' R' - Nu-H O Nu can either be negatively charged or neutral R R' - H O + Nu H Nu - H 2O R Nu-H R' Nu H Trends for Ranking Problems: In Nu- Addition reactions… Stability = Reactivity Aldehydes > Ketones More reactive Less reactive Aldehydes: Aliphatic > Aromatic (Steric and Electronic Effects) (Resonance effect) R R' Nucleophilic Addition Reactions 1. Hydration Base-catalyzed: O - O H - OH R R' Aldehyde or Ketone R H O OH R R' OH R' - + OH OH Acid-catalyzed: + O OH + H O H H R' R OH H2O R OH R R O H + R + R H2O + OH R H H3O 2. Cyanohydrin Formation: O - - C O HO CN N HCN H H CN + -C H N 3. Grignard Reagent: MgX + O R H + O - MgX :R :R and ethe r R MgX O H3O - + HOMgX R R H H OH + R - H R' 4. 1 Amines: Imine R' O :NH2R' R H O - OH H R + N H H R' H transfer H3O H R H N R' + + O H R' + N H H2O H R H R N R' N H H + H3 O R H Imine + 5. 2 Amines: Enamine O H C OH H C :NHR2 H H H R2N O + H + R + N H H C + C C H R N H2O H H C R R H H3O C R2 N Enamine 6. Wolff-Kishner (Hydrazine addition): To convert aldehydes and ketones into alkanes O H2NNH2 R R' H H + C KOH R + N2 H2O R' 7. Adding Alcohols: (Acetals) O + R Acid catalyst 2 R"OH R"O ( i.e. HCl ) R' OR" R + H2O R' 8. Wittig: O + P(Ph) 3 C C R - O C R' - + P(Ph) 3 O P(Ph) 3 C C C R' R R' R R' C + (Ph)3P C R O +