Aldehydes and Ketones Why are acetals useful as protecting groups
advertisement

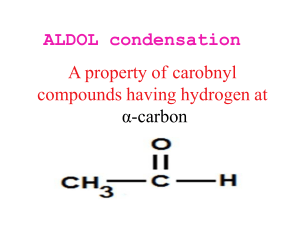
Aldehydes and Ketones Why are acetals useful as protecting groups for aldehydes and ketones? Describe the alpha halogenation of aldehydes and ketones. How is the reaction different if it is carried out in base instead of acid? What does cyanohydrin formation have in common with nucleophilic addition of a Grignard reagent to an aldehyde or ketone? Why is use of the Wittig reagent to prepare alkenes better than other methods to make alkenes that you have learned previously? Describe three ways to synthesize aldehydes or ketones, at least one of them new. What conditions or reagents encourage an aldol condensation product to undergo dehydration to form an enone? How is an enamine different from an imine? Describe how these two types of compounds are synthesized. Why are hydrogens alpha to carbonyl groups relatively acidic? How is an intramolecular aldol condensation different from a regular aldol condensation? Describe two kinds of reactions that aldehydes can undergo.