Chemical-Nomenclaturen-revised-w-hydrates

Chemical Nomenclature
according to
IUPAC the
International Union of Pure and Applied Chemistry
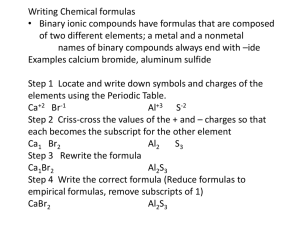
Binary Ionic Compounds
Made of 2 element
Metal bonded to nonmetal
Metallic cations have the same name as the metal
If a metal can form different oxidation states use a
Roman Numeral, known as
Stock System, following the metal name to indicate the oxidation state.
Or the Latin System using a
Latin root and suffix.
Lower oxidation state – ous
Higher oxidation state – ic
Nonmetal anions:
drop the ending and add –ide.
The Rule:
Unchanged cation name + anion name (drop ending) + ide
Examples:
NaCl: Sodium chloride
CaBr
2
: Calcium bromide
Multiple oxidation states
FeS:
Iron (II) sulfide
or Ferrous sulfide
Fe
2
S
3
:
Iron (III) sulfide
Ferric sulfide
Other Elements that use the Latin
System
Iron (ferrous and ferric)
Lead (plumbous and plumbic)
Mercury (Mercurous and Mercuric)
Nickel (Nickelous and Nickelic)
Chromium (Chromous and Chromic)
Manganese (Manganous and Manganic)
Cobalt (Cobaltous and Cobaltic)
Tin (Stannous and Stannic)
Antimony (Antimonious and Antimonic)
Copper (Cuprous and Cupric)
Binary Covalent Compounds
Made of 2 elements
2 Nonmetals bonded
Element farthest to the left in the
Periodic Table is the positive oxidation state.
Prefix to indicate number of atoms
+ unchanged nonmetal with the positive oxidation state + prefix to indicate number of atoms + nonmetal with negative oxidation state (drop ending) + ide.
Rule of double vowel contraction:
Drop the -a or -o before an -a or – o.
Keep all other double vowels.
Example: Pentoxide not pentaoxide
Example: Diiodide not diodide
Examples:
CO
2
: Carbon dioxide
N
2
0
5
: Dinitrogen pentoxide
Prefixes:
Mono-
Di-
Tri-
Tetra-
Penta-
Hexa-
Hepta-
Octa-
Nona-
Deca-
Enna-
Dodeca-
Ternary Compounds
Made of 3 or more elements
Ternary compounds – compounds of polyatomic ions
Polyatomic ions – ions made of more than one atom
The Rule:
Cation name + anion name.
Examples:
KNO
3
Postassium nitrate
Rb
2
SO
4
Rubidium sulfate
Metals with multiple oxidation states
Pb
3
(PO
3
)
2
Lead (II) phosphite
Or Plumbous phosphite
Pb
3
(PO
3
)
4
Lead (IV) phosphite
Or Plumbic phosphite
Binary Acids
Made of 2 elements (hydrogen and one other element)
Acid – hydrogen compounds of an monoatomic anion in water solution
Binary acids – acids made of two elements
Acids whose names end in –ide
Rule:
Add hydro to the beginning of the nonmetal’s name drop the ending and add –ic acid.
Hydro (nonmetal root) ic acid
Examples:
HCl
Hydrogen chloride (if pure substance) = hydrochloric acid (in water)
H
2
S
Hydrogen sulfide (if pure substance) = hydrosulfuric acid (in water)
Ternary Acids
Ternary acid – hydrogen compounds of polyatomic anions in water solution
Acids whose names end in –ite or –ate
Rule:
If the ending is –ite, replace it with –ous acid
If the ending is –ate, replace it with –ic acid\
Do not use hydro- at the beginning
Examples:
H
2
SO
4
HNO
2 is sulfuric acid not hydrosulfuric acid is nitrous acid not hydronitrous acid
Polyatomic Oxoanion Rules
Oxygen can form a series of polyatomic oxoanions formed from nonmetals and end in –ite and –ate
Example:
ClO -1 hypochlorite (assigned to lowest oxidation state of nonmetal)
ClO
2
ClO
3
-1
-1 chlorite chlorate
ClO
4
-1 perchlorate (assigned to highest oxidation state of nonmetal)
Series oxoanions: (usually)
If 1 in the series use -ate
If 2 in the series use -ate and –ite
If 3 in the series use -ate, -ite, and per___ate
If 4 in the series use -ate, -ite, per___ate, and hypo____ite
An additional series of ions can be formed by adding hydrogen to oxoanoins
Examples
PO
HPO
H
2
4
-3
PO
4
-2
4
-1
Phosphate ion
Monohydrogen phosphate ion or hydrogen phosphate ion
Dihydrogen phosphate ion or bihydrogen phosphate
Other Polyatomic Ion Rules
Some polyatomic ions end in –ide by convention
OH -1 hydroxide
CN -1 cyanide
N
3
-1 azide
Polyatomic ions of the same element
Add per-
O -2 is oxide
O
2
-2 is peroxide
S -2 is sulfide
S
2
-2 is persulfide
Polyatomic cations formed from nonmetals end –ium
Examples
NH
4
H
3
+1 – Ammonium
0 +1 – Hydronium
Hydrates
Compounds with water molecules surrounding the formula unit of the compound.
Rule:
Regular name + Prefix • hydrate
Example: MgSO
4
• 7 H
2
Magnesium Heptahydrate
O
Some Have Traditional Names by
Convention
H
2
O – Water
PH
3
NH
3
– Phosphine
– Ammonia
N
2
H
4
– Hydrazine
NO – Nitric Oxide
N
2
O – Nitrous Oxide