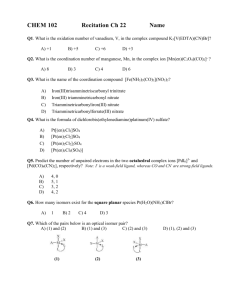
Naming Ternary
Compounds
Pisgah High School
M. Jones
Binary compounds have only
two kinds of atoms.
Ternary compounds
have three different
elements and contain a
polyatomic ion.
Naming compounds
containing polyatomic ions
1. Write the name of the metal
2. Add a Roman numeral, if
necessary
3. Write the name of the
polyatomic ion.
Polyatomic Ion List
This is the list of
polyatomic ions
that is found in
the NC Reference
Tables.
Polyatomic Ion List
This is a larger
list which has
been provided to
you.
Polyatomic Ion List
The first two columns are
the names and formulas
in alphabetical order by
the name.
The last two columns are
the formulas and names
in alphabetical order by
the formula.
Write the name for KClO3
Write the name of the metal
Potassium does not require a Roman
numeral since it has only one
oxidation number and is group IA.
Then add the name of ClO3
1-
potassium chlorate
Write the name of Fe2(SO4)3
Since iron has two possible
oxidation numbers, we will need
a Roman numeral in the name.
To determine the oxidation
number of iron, we must start
with sulfate.
Write the name of Fe2(SO4)3
The parentheses and the subscript
tell us that there are three sulfates.
2-,
Since sulfate, SO4 has an
oxidation number of -2, we can
determine that iron has an oxidation
number of +3.
Write the name of Fe2(SO4)3
? +
+6
+6 / 2
+3
(-6) = 0
-6
-2 x 3
-2
Fe2(SO4)3
Write the name of Fe2(SO4)3
? +
+6
+6 / 2
+3
(-6) = 0
-6
-2 x 3
-2
Fe2(SO4)3
Write the name of Fe2(SO4)3
+3
The oxidation
number of iron
is +3.
Fe2(SO4)3
Write the name of Fe2(SO4)3
Write the name of the metal
Add the Roman numeral that
equals the oxidation number.
Write the name of the polyatomic
2ion, SO4 .
iron(III) sulfate
Write the formula for copper(II) nitrate
Write the symbols for copper and
nitrate.
The
Roman
numeral
Criss-cross
tells the oxidation
to get the
number of copper.
formula.
Nitrate is –1.
+2
-1
Cu(NO3 )2
Write the names of the following:
1. KNO3
Potassium nitrate
2. CuSO4
Copper(II) sulfate
3. Fe(ClO3)3 Iron(III) chlorate
4. Sn3(PO4)2 Tin(II) phosphate
5. (NH4)2CO3 Ammonium carbonate
Write the formulas of the following:
1. Lithium carbonate
Li2CO3
2. Barium hydroxide
Ba(OH)2
3. Gold(III) sulfate
Au2(SO4)3
4. Ammonium chloride
NH4Cl
5. Chromium(II) nitrate
Cr(NO3)2
Mike Jones
Pisgah High School
Canton NC
mjones@haywood.k12.nc.us
Copyright 2012
All rights reserved.