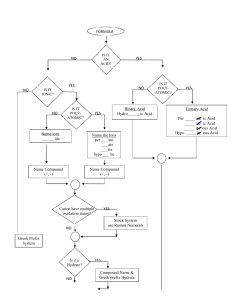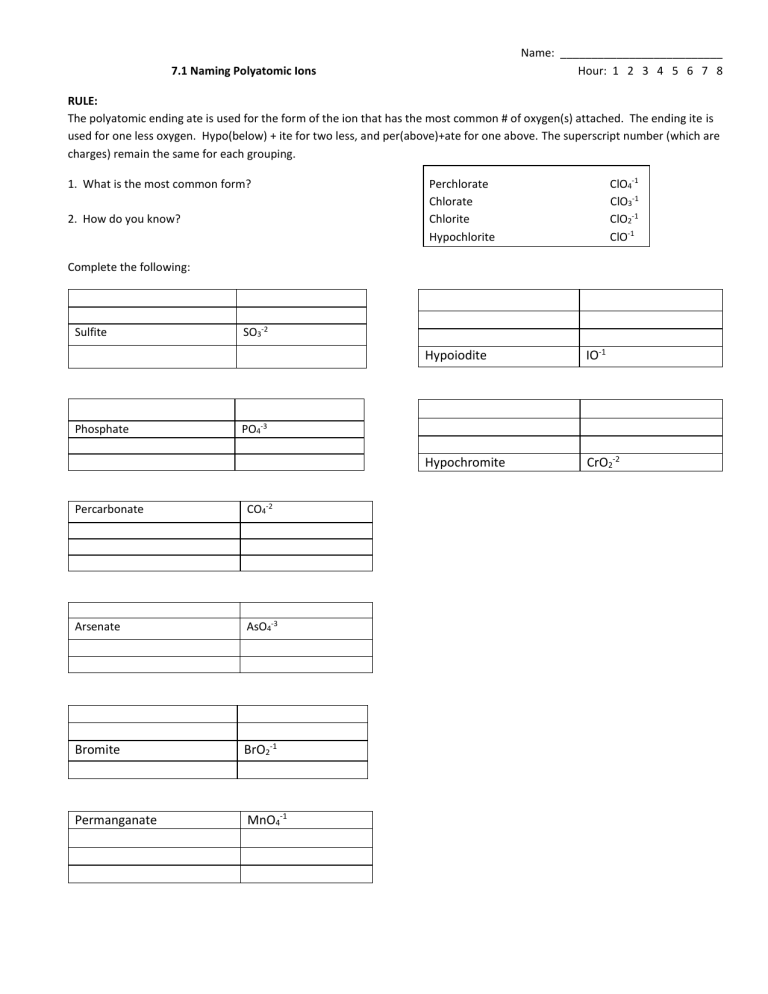
Name: __________________________ Hour: 1 2 3 4 5 6 7 8 7.1 Naming Polyatomic Ions RULE: The polyatomic ending ate is used for the form of the ion that has the most common # of oxygen(s) attached. The ending ite is used for one less oxygen. Hypo(below) + ite for two less, and per(above)+ate for one above. The superscript number (which are charges) remain the same for each grouping. 1. What is the most common form? 2. How do you know? ClO4-1 ClO3-1 ClO2-1 ClO-1 Perchlorate Chlorate Chlorite Hypochlorite Complete the following: Sulfite Phosphate SO3-2 Hypoiodite IO-1 Hypochromite CrO2-2 PO4-3 Percarbonate CO4-2 Arsenate AsO4-3 Bromite BrO2-1 Permanganate MnO4-1