Concept Map #8 - Writing Formula, Naming Compounds, GFM
advertisement

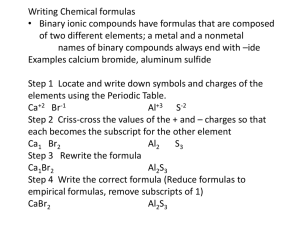
VOCABULARY Polyatomic Ion Chemical Formula Empirical Formula Structural Formula Binary Compound Ternary Compound Ion Charge Oxidation Number Subscript Superscript Diatomic Element Atomic Mass Atomic Mass Unit Formula Mass Molecule Gram-Formula Mass Molar Mass Mole WRITING CHEMICAL FORMULAS NAMING COMPOUNDS GRAM-FORMULA MASS Essential Questions 1. What is the shorthand method used to represent elements and compounds? 2. How is a mole used to ‘count’ atoms? Essential Knowledge 1. The IUPAC system gives rules for naming & writing chemical formulas for compounds. 2. The formula mass of a substance is the sum of the atomic masses of all the atoms in its chemical formula. The units for formula mass are atomic mass units (amu). 3. The molar mass or gram-formula mass of a substance is the mass in grams of one mole of that substance. The units for gram-formula mass are grams (g). 4. One mole of a substance contains 6.02 x 1023 particles. Types of Chemical Formulas Molecular Formula Empirical Formula Structural Formula Types of Compounds Binary Compounds Ternary Compounds Table E Polyatomic Ions Rules for Writing Chemical Formulas 1. List the symbol of each element and/or polyatomic ion Table S lists element names & symbols Table E lists the polyatomic ions Always put ( ) around the polyatomic ions 2. List oxidation numbers (ion charges) as superscripts If an element has more than one oxidation number, use the top one listed on the periodic table unless a Roman number is present Ion charges for the polyatomic ions are on Table E 3. Criss-cross the oxidation numbers so they become the subscripts Drop + and - signs Drop subscripts of ‘1’ Drop ‘equal’ subscripts (+2 and -2 or +3 and -3) Drop the ( ) only IF there is a subscript of ‘1’ outside of it 4. Re-write answer and box it in Diatomic Elements H2 O2 F2 Br2 I2 N2 Cl2 Counting Atoms Formula Mass vs Gram-Formula Mass (Molar Mass) Rules for Naming Compounds 1. Binary Compounds (only contain two different elements) Name the first element Name the second element by change its ending to –ide 2. Ternary Compounds (contain three or more different elements) Use Table E to name the polyatomic ion If the element on the right end is not part of the polyatomic ion, name it by changing its ending to –ide.