Chemical Formula Writing & Nomenclature Binary Acids Naming
advertisement

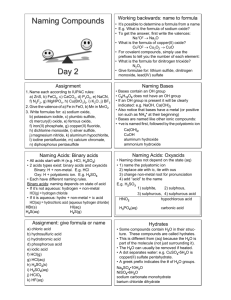
© Mr. D. Scott; CHS Chemical Formula Writing & Nomenclature Binary Acids Ternary Acids Basic Rules for acids All formulas begin with hydrogen, H Those hydrogens listed first are the ones capable of being ionized when in solution and/or reacted with bases. Hydrogens listed later in the formula are not capable of being ionized. Binary Acids These follow the format HxAy where A represents some non-metal element covalently bonded to hydrogen. Naming Binary Acids hydro-ic add prefix root of non-metal name acid This naming approach assumes that the acid compound in aqueous (dissolved in water). When these compounds are pure, the naming rules follow those of the binary molecules that we will cover later. Until further notice, we will name these compounds as acids in aqueous solution. © Mr. D. Scott; CHS The oxidation state of the non-metal follows the same rule as it does when it is combining with a metal. It will be the first one listed and negative. Examples: H+P H+1 + P-3 H3P hydrophosphoric acid H+S H+1 + S-2 H2S H + Se H H+F +1 + Se-2 H2Se H+1 + F-1 HF hydroselenic acid hydrofluoric acid + Cl-1 HCl hydrochloric acid H + Br H+1 + Br-1 HBr hydrobromic acid H+I H+1 + I-1 hydroiodic acid H + Cl H +1 hydrosulfuric acid HI © Mr. D. Scott; CHS Ternary Acids These follow the format Hx(polyatomic ion)y An important consideration – All acids are molecules. While we are using our PAL to combine various polyatomic ions with hydrogen, these are NOT IONIC COMPOUNDS. The hydrogen is covalently bonding somewhere on the polyatomic ion and producing a molecular substance. Naming Ternary Acids root of polyatomic name -ate -ic -ite -ous acid The ending of the polyatomic name is changed when naming these acids as indicated above. The rest of the polyatomic name is not changed. Not every polyatomic ion will make an acid when combined with hydrogen. Your knowledge about these will be gained through experience. © Mr. D. Scott; CHS Examples: H + nitrate HNO3 nitric acid H + nitrite HNO2 nitrous acid H + hypochlorite HClO hypochlorous acid H + chlorite HClO2 chlorous acid H + chlorate HClO3 chloric acid H + perchlorate HClO4 perchloric acid H + acetate HC2H3O2 acetic acid H + carbonate H2CO3 carbonic acid H + oxalate H2C2O4 oxalic acid H + citrate H3C6H5O7 citric acid