Enolates
advertisement

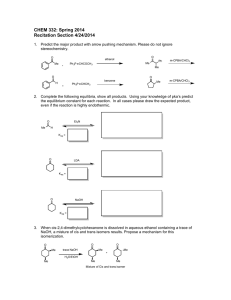
CHAPTER TWENTY-TWO: ENOLS AND ENOLATES 1. Formation of enolates a. pKa Aldehyde (≈ 18), Ketone(≈20) and Ester(≈22) -ketoester (≈ 11) b. hydroxide, alkoxide, LDA comparison 2. Keto-Enol Tautomerism (review) a. Mechanism in acid (Mechanism 22-5) b. Mechanism in base (Mechanism 22-4) LDA vs RO– as base Kinetic vs thermodynamic control of LDA reaction Alkylation of Ketones via Enolate (LDA then 1° RX) Enamines a. Regioselective formation of less hindered Enamine b. Mechanism of formation from Ketone via Iminium Ion c. SN2 Alkylation with unhindered alkyl halides, -haloketones and -haloesters d. Acylation with acid chlorides and anhydrides to form -ketoketones 7. Halogenation of Ketones a. In acidic solution: Monohalogenation (reactant ketone more reactive than product ketone): Mechanism 22-8 b. In basic solution: Polyhalogenation (product more reactive than reactant): Mechanism 22-6 c. Haloform reaction of methyl ketones (Mechansim 22-7) 8. Halogenation of Carboxylic Acids (Section 22-6) Hell-Volhard-Zelinsky Rxn: Br2 with PBr3 (Mechanism pg 1060) 3. 4. 5. 6. 9. The Aldol Condensation (carbonyl condensation of ketones and aldehydes) i. Mechanism in acid and base (Mechanism 22-9 & 22-10) ii. Self condensation iii. Elimination (Section 22-8) iv. Crossed (limitations: multiple products) (Section 22-9) v. Intramolecular: Cyclizations (Section 22-10) vi. Reversibile: experimental consequences 10. The Claisen Condensation: Formation of -ketoester products a. Mechanism (Mechanism 22-12) b. Subsequent decarboxylation (Mechanism pg 1080) c. Crossed Claisen: Only one ester can have - H’s (Section 22-14) d. Variation: Ketone nucleophile + ester electrophile 11. Malonic Ester Synthesis: Using malonic ester (CH2(COOEt)2) with NaOEt then RX to make -alkylated carboxylic acids (RCH2COOH) Mechanism: -alkylation, saponification, acidification, decarboylation 12. Acetoacetic Ester Synthesis: Using ethyl acetoacetate (CH3COCH2COOEt) with NaOEt then RX to make -alkylated methyl Ketones (CH3COCH2R) Mechanism: -alkylation, saponification, acidification, decarboylation 13. Micheal Addition : Conjugate addition of stabilized enols, enamines or Gilman reagents to -unsaturated carbonylsformation of -dicarbonyl: (Table 22-2; Section 22-18) a. Conjugate vs carbonyl addition as function of reversibility b. Grignard/ Organolithium: carbonyl addition c. Water/Amines/cyanide stabilized carbon nucleophiles: Conjugate addition 14. Robinson Annulation: Michael (Conjugate) Addition then intramolecular Aldoldehydration to form -unsaturated cyclic ketone (Section 22-19) LEARNING OUTCOMES: Predict the missing product and/or reactant in a relevant chemical reaction involving carboxylic acid derivatives. Use curved-arrow formalism to depict the known mechanism of an organic reaction. Devise multi-step syntheses of molecular targets using comprehensive knowledge of functional group transformations. Understand the structural features that relative reactivity in acyl transfer reactions (interconversion of carboxylic acid derivatives via addn- elimination reactions) SAMPLE EXAM PROBLEMS: 1. Propose a sequence of steps to effect the following chemical transformation in high yield: 2. Rank the acidity of the following Bronsted acids from strongest acid (1) to weakest acid (4)