CHEM 332: Spring 2014 Recitation Section 4/24/2014
advertisement
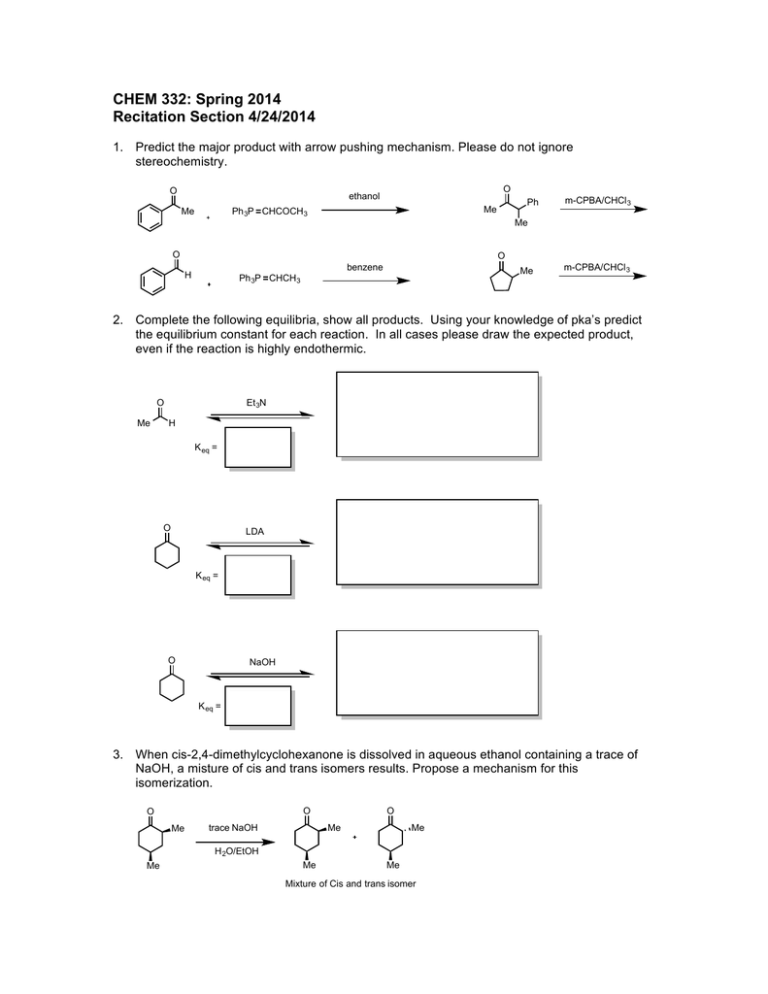
CHEM 332: Spring 2014 Recitation Section 4/24/2014 1. Predict the major product with arrow pushing mechanism. Please do not ignore stereochemistry. O O ethanol Me Ph Me Ph 3P CHCOCH3 m-CPBA/CHCl 3 Me O O benzene H Me Ph 3P CHCH3 m-CPBA/CHCl 3 2. Complete the following equilibria, show all products. Using your knowledge of pka’s predict the equilibrium constant for each reaction. In all cases please draw the expected product, even if the reaction is highly endothermic. O Me Et 3N H K eq = O LDA K eq = O NaOH K eq = 3. When cis-2,4-dimethylcyclohexanone is dissolved in aqueous ethanol containing a trace of NaOH, a misture of cis and trans isomers results. Propose a mechanism for this isomerization. O O Me O Me trace NaOH Me H 2O/EtOH Me Me Me Mixture of Cis and trans isomer 4. When bromo ketone 1 is treated with potassium tert-butoxide in tert-butyl alcohol at room temperature, it gives exclusively 5,5-fused bicyclic ketone 2. In contrast, when 1 is treated with LDA in THF at -72 °C, followed by heating, the product is predominately the 5,7 fused ketone 3. Write arrow formalism mechanisms for these cycloalkylation reactions and explain why the different reaction conditions favor different products. O Me Br 1 1. KOt-Bu/ t-BuOH, 25 °C 2. H 2O O 1. LDA/THF, - 72 °C 2. Δ 3. Η2Ο Me 2 O 3 5. Provide simple synthetic routes from cyclohexanone to the following compounds: O O Me O Br D D D D O 6. Give the expected products for the aldol condensations of (a) propanal (b) pentan-3-one











