1
ChemQuest 88
Name: ____________________________
Date: _______________
Hour: _____
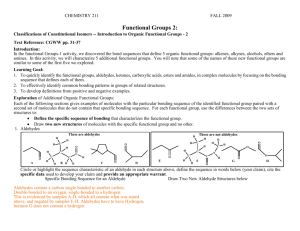
Information: Ethers and Alcohols
So far, you have learned about alkanes and alkenes, which are composed of only carbon and
hydrogen atoms. Some organic compounds contain an oxygen atom with only single bonds. Any
such compound would either be an ether or an alcohol. Examine a couple ethers and alcohols below:
Ethers
Alcohols
Critical Thinking Questions
1. Both alcohols and ethers contain single-bonded oxygen atoms. Compare the ethers and the
alcohols carefully. What is the difference between an alcohol and an ether?
They both have single-bonded oxygen atoms, but the oxygen in alcohols have a
hydrogen atom bonded to it.
2. Label each of the following as an ether or an alcohol.
ether
__________________________
ether
_________________________
alcohol
__________________________
alcohol
_________________________
Copyright 2002, 2014 by Jason Neil. All rights reserved.
To make copies permission must be obtained from www.ChemistryInquiry.com.
2
Information: Ketones and Aldehydes
Ketones
Aldehydes
Critical Thinking Questions
3. What is similar about ketones and aldehydes?
They both have a carbon-oxygen double bond.
4. Look at the ketones and aldehydes carefully. How are they different?
With an aldehyde, the carbon that contains the double-bonded oxygen has a hydrogen
bonded to it. The same carbon lacks a hydrogen in ketones.
5. Label each of the following as either a ketone or an aldehyde.
ketone
__________________________
aldehyde
_________________________
ketone
__________________________
ketone
_________________________
Copyright 2002, 2014 by Jason Neil. All rights reserved.
To make copies permission must be obtained from www.ChemistryInquiry.com.
3
Information: Esters and Carboxylic Acids
Esters
Carboxylic acids
Critical Thinking Questions
6. How are esters and carboxylic acids similar?
They both have a carbon-oxygen double bond AND another oxygen with single bonds.
7. How are esters and carboxylic acid different?
Carboxylic acids have a hydrogen bonded to their single-bonded oxygen atoms, but
esters do not.
8. Label each of the following as either an ester or a carboxylic acid.
ester
__________________________
Carboxylic acid
_________________________
Carboxylic acid
__________________________
ester
_________________________
Copyright 2002, 2014 by Jason Neil. All rights reserved.
To make copies permission must be obtained from www.ChemistryInquiry.com.
4
9. Label each molecule as a ketone, alcohol, ether, ester, carboxylic acid, or aldehyde.
ether
__________________________
alcohol
_________________________
ketone
__________________________
ester
_________________________
Carboxylic acid
__________________________
ester
_________________________
aldehyde
__________________________
ether
_________________________
Copyright 2002, 2014 by Jason Neil. All rights reserved.
To make copies permission must be obtained from www.ChemistryInquiry.com.
5
Copyright 2002, 2014 by Jason Neil. All rights reserved.
To make copies permission must be obtained from www.ChemistryInquiry.com.