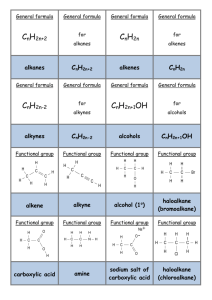
Summary Sheet - Introduction to Chemical Reactivity, Nomenclature
advertisement
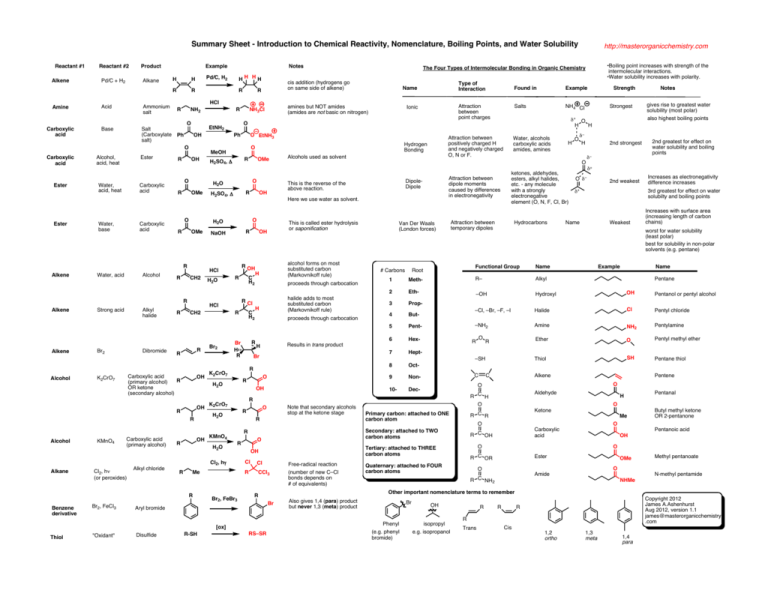
Summary Sheet - Introduction to Chemical Reactivity, Nomenclature, Boiling Points, and Water Solubility Reactant #1 Alkene Amine Carboxylic acid Reactant #2 Pd/C + H2 Product Alkane Acid Ammonium salt Base Example H H R R R R NH2 Salt (Carboxylate Ph salt) O Ester Ester Alcohol, acid, heat Ester Water, acid, heat Carboxylic acid Water, base Carboxylic acid OH R O R O R Water, acid Alcohol CH2 Alkene Alcohol Strong acid Alkyl halide Br2 Dibromide R R R OH OH Carboxylic acid (primary alcohol) R Alkane Cl2, hν (or peroxides) R Benzene derivative Br2, FeCl3 C H2 C H2 proceeds through carbocation halide adds to most substituted carbon (Markovnikoff rule) H R Br H R proceeds through carbocation Br K2CrO7 OH R KMnO4 O R Cl R Br2, FeBr3 Cl "Oxidant" CCl3 R-SH (number of new C–Cl bonds depends on # of equivalents) Br δ+ O δ− 2nd weakest Increases as electronegativity difference increases 3rd greatest for effect on water solubilty and boiling points δ+ Name Weakest Increases with surface area (increasing length of carbon chains) Name R– Alkyl 2 Eth- –OH Hydroxyl OH Pentanol or pentyl alcohol 3 Prop- 4 But- –Cl, –Br, –F, –I Halide Cl Pentyl chloride 5 Pent- –NH2 Amine NH2 Pentylamine 6 Hex- O Ether O Pentyl methyl ether 7 HeptThiol SH Pentane thiol 8 Oct- 9 Non- R R C C Example Dec- Alkene Pentene O Aldehyde R C H O R C R O Secondary: attached to TWO carbon atoms R C OH H Pentanal O Ketone Me Butyl methyl ketone OR 2-pentanone O Carboxylic acid Pentanoic acid OH O O R C OR Quaternary: attached to FOUR carbon atoms Name Pentane O Primary carbon: attached to ONE carbon atom Ester OMe Methyl pentanoate O O Amide R C NH2 Also gives 1,4 (para) product but never 1,3 (meta) product Br Phenyl RS–SR 2nd greatest for effect on water solubility and boiling points N-methyl pentamide NHMe Other important nomenclature terms to remember R Aryl bromide Disulfide Free-radical reaction 2nd strongest δ− Functional Group Root Tertiary: attached to THREE carbon atoms OH [ox] Thiol Note that secondary alcohols stop at the ketone stage R H Meth- 10- O R O gives rise to greatest water solubility (most polar) also highest boiling points 1 # Carbons R K2CrO7 Strongest Notes worst for water solubility (least polar) best for solubility in non-polar solvents (e.g. pentane) –SH O R Van Der Waals (London forces) Hydrocarbons Strength δ− O H H ketones, aldehydes, esters, alkyl halides, etc. - any molecule with a strongly electronegative element (O, N, F, Cl, Br) Attraction between temporary dipoles Results in trans product H R Me R alcohol forms on most substituted carbon (Markovnikoff rule) H R Cl Cl2, hγ Alkyl chloride NH4 Cl Water, alcohols carboxylic acids amides, amines Attraction between dipole moments caused by differences in electronegativity DipoleDipole This is called ester hydrolysis or saponification OH R OH R H2O OH R H2O OH R Salts O This is the reverse of the above reaction. O NaOH Br2 Alcohols used as solvent Here we use water as solvent. H2O R KMnO4 R H2SO4, Δ CH2 R Alcohol OMe O HCl Carboxylic acid R (primary alcohol) OR ketone (secondary alcohol) K2CrO7 R H2SO4, Δ H2O Example δ+ Attraction between positively charged H and negatively charged O, N or F. Hydrogen Bonding O HCl R Alkene O EtNH3 H2O OMe R Ph H2O OMe Attraction between point charges Ionic Found in H MeOH OH amines but NOT amides (amides are not basic on nitrogen) NH3Cl Type of Interaction Name O EtNH2 R Alkene R •Boiling point increases with strength of the intermolecular interactions. •Water solubility increases with polarity. The Four Types of Intermolecular Bonding in Organic Chemistry cis addition (hydrogens go on same side of alkene) R HCl O Carboxylic acid Notes HH HH Pd/C, H2 http://masterorganicchemistry.com (e.g. phenyl bromide) OH isopropyl e.g. isopropanol R R Copyright 2012 James A.Ashenhurst Aug 2012, version 1.1 james@masterorganicchemistry .com R R Trans Cis 1,2 ortho 1,3 meta 1,4 para