Traditional Synthesis vs Green Chemistry
advertisement

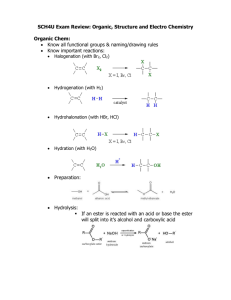
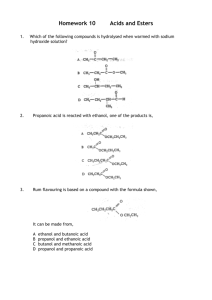
Group H Dyshuwnna Nobles, Austin Saunders, Jamil Rogers A LITTLE TASTE OF CHEMISTRY A WHIFF OF WHAT IT’S ALL ABOUT DID YOU CATCH THAT WHIFF? Perfumes Deodorants Candy Beverages Food Fragrance and Flavor Chemistry plays a vital role in our everyday lives!!! WHAT’S THAT SMELL Ester functional groups are widely studied in the field of organic chemistry Low molecular weight often have a pleasant smell fruit floral candy INTRODUCTION We are exploring different syntheses and extractions to create an ester. Our main goal was to improve the protocol on creating the ester. The protocol was meant to improve the yield of many esters through combinatorial chemistry. This experiment will be used in the undergraduate organic chemistry lab. FISCHER ESTERIFICATION Fischer Esterification is the process in which an alcohol and a carboxylic acid are combined and form an ester. Combinations we MADE!!!!!! 2 Methyl 1 propanol Formic Acid Propionic Acid Phenylacetic Acid Acetic Acid 3 methyl-1 butonal 1 propanol 1 butonal INSTRUMENTS These are the instruments that we used to analyze our data Nuclear Magnetic Resonance Gas Chromatography Infrared Spectrometer PROCEDURES 1. We used Fischer Esterification 1. We measured out our carboxylic acids by their mass and our alcohol by their volume 2. We used 2 moles of alcohol for every 1 moles of carboxylic acid 3. Avagadro’s number is the number used in a mole. It is the amount of how many molecules are in a mole. The number is 6.022142*10(23) Heating Process…. We heated our mixture for an hour Washing of our product…… We washed our mixture using biocarbonate to rid the substance of the sulfuric acid and carboxylic acid Result E A C D B 400 MHz NMR (Nuclear Magnetic Resonance) Sigma Aldrich Co E F A B C Austin’s ester -Tropical D F Infrared Spectrometer Austins Ester C-H bonds C =O C-O Jamil’s Ester Alcohol O-H bond C =O C-O Gas Chromatography RT: 0.00 - 30.01 8.97 100 NL: RT: 0.00 - 18.38 2.19E8 100 TIC F: MS SEECoS_tr opical 90 ESTER Tropical 90 80 2.95 NL: 4.82E7 TIC F: MS SEECoS_J amil_C3 EsterRaspberry 80 70 Relative Abundance 70 60 50 40 60 50 40 30 30 20 20 2.82 10 10 2.58 2.65 3.90 7.98 8.29 10.28 11.29 13.45 17.03 18.63 20.78 0 0 5 Austin’s 10 15 Time (min) 20 24.33 27.73 29.75 25 30 2.60 3.75 0 0 2 4 5.63 6.22 7.93 8.44 10.89 11.50 12.54 6 8 10 Time (min) Jamil’s 12 14.93 16.81 17.03 14 16 18 CONCLUSION We were able to create an ester using different synthesis and extractions. Further Research If water washes don't get rid of the alcohol, we might have to include distillation. Dr. Jackie Bortiantyski- SEECOS Professor Kaitlyn Sanders- Graduate Student SEECOS Faculty Jody Markley- Director Derek James- Assistant Director Joanne Nash- administrative Coordinator Jackie Mills- Group Mentor UBMS Staff Thank you Works Cited •http://www.mynewsletterbuilder.com/ex/template_content_corner/ex82/images/fragrance %2520sticks%2520general.jpg http://www.novelguide.com/a/discover/plsc_02/plsc_02_00137.html