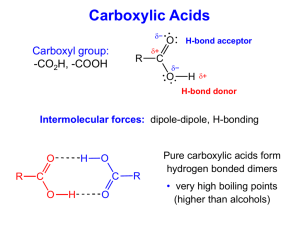
Carboxylic Acid Questions 1.-Arrange the following in order of (i
advertisement

Carboxylic Acid Questions 1.-Arrange the following in order of (i) increasing acidity, (ii) increasing pKa : (a) CH3CO2H (b) CCl3CO2H (d) CH3CH2SH (c) CH3CH2OH 2.-What would be the major products of the reactions of (i) butanoic acid and (ii) benzoic acid with each of the following: (a) SOCl2, Et3N (c) (CH3)2CHOH / H+ / heat (b) LiAlH4 / THF then acidic work-up (d) NaOH 3.-How could you use 1-bromobutane to prepare each of the following carboxylic acids ? (a) propanoic acid (b) butanoic acid (c) pentanoic acid (d) hexanoic acid Carboxylic Acid Answers 1: (i) increasing acidity : CH3CH2OH < CH3CH2SH < CH3CO2H < CCl3CO2H Resonance stabilization in carboxylates makes carboxylic acids more acidic than alcohols or thiols. Electron withdrawing groups, here -Cl, increase the acidity due to further stabilization of the carboxylate. Thiols are more acidic than alcohols due to weaker X-H bond and the ability of S to accommodate extra electrons (size). (ii) increasing pKa : CCl3CO2H < CH3CO2H < CH3CH2SH < CH3CH2OH Remember the lower the pKa the stronger the acid, so once you have part (i), this is just the opposite. Tip: When asked about pKa trends, it may be easier to think in terms of acidity then remember to flip the order. 2: Note the identical nature of the reactions that the aliphatic and aromatic acid undergo: (a) Thionyl chloride, SOCl2, is used to prepare acyl chlorides, the base removes the HCl by-product. (b) LiAlH4 is a hydride reducing agent, acids to primary alcohols. (c) An alcohol and a carboxylic acid give an ester. (d) Carboxylic acids react with bases to give carboxylates. 3: First, note that we have an homologous series of C3 to C6 acids we are trying to make. Here is a scheme collecting possible syntheses together (based on the more important reactions) (a) Propanoic acid : need to lose a C from C4. We can do this via ozonolysis of an alkene, which we can obtain by eliminating the alkyl halide. (b) Butanoic acid : oxidation of the corresponding alcohol will give the carboxylic acid, so prepare the alcohol by substitution. (c) Pentanoic acid : need to gain a C to get C5. One way to do this is via the reaction of the Grignard reagent with carbon dioxide. Alternatively, substitution with NaCN then hydrolysis would also work. (d) Hexanoic acid : need to gain 2C to get C6. Reaction of the Grignard reagent with ethylene oxide gets the right number of C and a primary alcohol ready for oxidation to the acid. More questios….. 4: Arrange the following in order of decreasing reactivity towards hydrolysis using aqueous NaOH. (a) CH3CO2CH3 (c) CH3CON(CH3)2 (b) CH3COCl (d) CH3CO2COCH3 5: What are the products of the hydrolysis reactions considered in Qu 1 after a dilute acid work-up ? 6: (a) Arrange the following in order of decreasing reactivity towards LiAlH4 / THF followed by dilute acid work-up. (b) What are the products of each of the reactions in part (a) ? 7: What is the product of each of the following reactions ? Answers: 4 Hydrolysis is a nucleophilic acyl substitution reaction, typical of carboxylic : acid derivatives. First task should be to identify the functional groups in each molecule then use the reactivity order. It can be rationalised based on (i) the interaction of the substituent and the carbonyl group, and, (ii) the ability of the substituent to function as a leaving group. CH3COCl CH3COOCOCH3 CH3COOCH3 CH3CON(CH3)2 > > > acid anhydride ester amide chloride 5 All the carboxylic acids in Qu 1 are derivatives of ethanoic acid, so they all : give the same carboxylic acid... (a) CH3CO2H (c) CH3CO2H and CH3OH (b) 2 moles CH3CO2H (d) CH3CO2H and HN(CH3)2 6 LiAlH4 is a source of H- (a nucleophile) which functions as a reducing agent. : First task should be to identify the functional groups: carboxylic acid, ketone, aldehyde, ester. The aldehyde and ketone will undergo nucleophilic addition, the acid and the ester nucleophilic acyl substitution. Consider the electrophilicity of the carbonyl group in each compound in each pair. Aldehydes are more reactive than ketones !!! as they are less hindered and the alkyl group in the ketone is a weak electron donor. Under the reaction condition s the carboxylic acid will deprotonate to give the carboxylate which is a very poor electrophile (after all, it has a negative charge !) so the ester is more reactive than the acid. Now combine the two pairs. Since the -OR group is a stronger electron donor (resonance) than the alkyl group of the ketone, the ester is less reactive than the ketone... so we get : (b) The aldehyde, carboxylic acid and ester will be reduced to the same product, benzyl alcohol. The ketone will be reduced to 1-phenylethanol, C6H5CH(OH)CH3 7 The answers the these questions involve materials from this chapter and : review from chapters 10 and 19