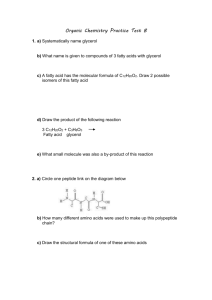
Pentane
advertisement

LAB: Constructing molecular models of organic compounds (include title on your lab report) PURPOSE: To practice visualizing and drawing structures for many organic compounds (include purpose as well) PROCEDURE: Construct molecular models for each of following organic compounds. After constructing the model, write its name and then draw a picture of it in your lab report, and write its formula. Also, answer relevant questions which appear below in your lab report. PART I 1) Pentane 2) 2-methylbutane 3) 2,2-dimethylpropane In your lab report: What is significant about structures 1-3? Which one would have the highest boiling point? Why? 4) 5) 6) 7) 8) 3-ethylhexane cyclopentane 1,2-dichlorocyclohexane 2-bromo-4-iodo-1-butene 4,4-dimethyl-2-pentyne Hint: for #’s 9-12, see margin note on page 1033 of your textbook. 9) cis-2-pentene 10) trans-2-pentene 11) cis-1,2-dichloroethene 12) trans-1,2-dichloroethene In your lab report: Explain the difference between cis and trans alkenes. Why do cis and trans isomers not exist for alkanes? Why do cis and trans isomers not exist for alkynes? PART II Use your textbook and workbook to create models for #’s 13-26 13) 3-pentanol 14) 2,2-dimethyl-1-pentanol 15) butanoic acid 16) 3-methylbutanoic acid 17) hexanal 18) 2,3-dimethylhexanal 19) 2-pentanone 20) 3-ethyl-2-pentanone 21) methyl butanoate 22) ethyl 4-methylpentanoate 23) 2-ethoxybutane 24) 1-chloro-2-methoxyethane 25) 2-chloro-1-butanamine 26) 2-methylcyclobutanamine 27) propanamide 28) 2-methylbutanamide In your lab report: label #’s 13-28 as one of the following: ALCOHOL, ETHER, ESTER, ALDEHYDE, KETONE, CARBOXYLIC ACID, AMINE. AMIDE In your lab report: Create a table which summarizes the system used for naming each of the following: ALCOHOL, ETHER, ESTER, ALDEHYDE, KETONE, CARBOXYLIC ACID, AMINE, AMIDE PART II Use your textbook and workbook to create models for #’s 13-26 13) 3-pentanol 14) 2,2-dimethyl-1-pentanol 15) butnaoic acid 16) 3-methylbutanoic acid 17) hexanal 18) 3,4-dimethylhexanal 19) 2-pentanone 20) 3-ethyl-2- pentanone 21) methyl butanoate 22) ethyl pentanoate 23) 2-methoxybutane 24) ethoxyethane 25) methanamine 26) cyclobutanamine In your lab report: Use the chart on page 1052 of your textbook in order to label #’s 13-26 as one of the following: ALCOHOL, ETHER, ESTER, ALDEHYDE, KETONE, CARBOXYLIC ACID, AMINE. In your lab report: Write a brief description of the process used for naming each of the following: ALCOHOL, ETHER, ESTER, ALDEHYDE, KETONE, CARBOXYLIC ACID, AMINE.