Carboxylic Acids211
advertisement

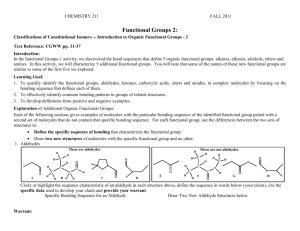
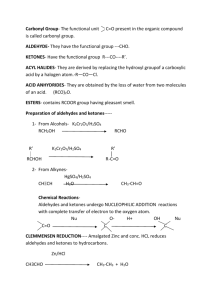
CHEMISTRY 211 FALL 2009 Functional Groups 2: Classifications of Constitutional Isomers -- Introduction to Organic Functional Groups - 2 Text Reference: CGWW pp. 31-37 Introduction: In the functional Groups-1 activity, we discovered the bond sequences that define 5 organic functional groups: alkenes, alkynes, alcohols, ethers and amines. In this activity, we will characterize 5 additional functional groups. You will note that some of the names of these new functional groups are similar to some of the first five we explored. Learning Goal: 1. To quickly identify the functional groups, aldehydes, ketones, carboxylic acids, esters and amides, in complex molecules by focusing on the bonding sequence that defines each of them. 2. To effectively identify common bonding patterns in groups of related structures. 3. To develop definitions from positive and negative examples. Exploration of Additional Organic Functional Groups: Each of the following sections gives examples of molecules with the particular bonding sequence of the identified functional group paired with a second set of molecules that do not contain that specific bonding sequence. For each functional group, use the differences between the two sets of structures to: Define the specific sequence of bonding that characterizes the functional group. Draw two new structures of molecules with the specific functional group and no other. 1. Aldehydes These are aldehydes H H H C H O A H C H B H H C O H H C D E C C O H C H C H H C C H O O H These are not aldehydes H H F O N C H O H O H G O O H Circle or highlight the sequence characteristic of an aldehyde in each structure above, define the sequence in words below (your claim), cite the specific data used to develop your claim and provide an appropriate warrant. Specific Bonding Sequence for an Aldehyde Draw Two New Aldehyde Structures below Aldehydes contain a carbon single-bonded to another carbon, Double-bonded to an oxygen, single-bonded to a hydrogen. This is evidenced by samples A-D, which all contain what was stated above, and negated by samples E-H. Aldehydes have to have Hydrogen, because G does not contain a hydrogen. 2 2. Ketones Func. Groups-2 These are ketones H H H C H H H C C C O H I C J H H K H C Cl N H H H O H C C L O H H O M O C H O H H H H O H C H These are not ketones H H C H O C N C C H O H P O Circle or highlight the sequence characteristic of a ketone in each structure above, define the sequence in words below (your claim), cite the specific data used to develop your claim and provide an appropriate warrant. Specific Bonding Sequence for a Ketone Draw Two New Ketone Structures below Ketones have a carbon single-bonded to another carbon, Which is double-bonded to an oxygen, which is bonded To another carbon. The structure creates a pyramidal shape. Samples I-L all contain the above-stated structure. Sample O Contains a nitrogen, which ketones do not contain. And sample N Contains a chloride, which ketones also do not have. 3. Carboxylic Acids These are not carboxylic acids These are carboxylic acids H H O H H H C C O Q O H C H H H C H H R O H O H O S H C H O H O O O T H U C H C C C H V O Br O H H N H W H O N X O H Circle or highlight the sequence characteristic of a carboxylic acid in each structure above, define the sequence in words below (your claim), cite the specific data used to develop your claim and provide an appropriate warrant. Specific Bonding Sequence for a Carboxylic Acid Draw Two New Carboxylic Acid Structures below Carboxylic acids have a central carbon single-bonded to another Carbon on one side, and then branched to contain a double-bond to oxygen, Which is single- bonded to another oxygen, which us bonded to hydrogen. Func. Groups-2 3 This is evidenced by samples Q-T. Example U has an extra carbon between the two oxygens, which does not constitute a carboxylic acid. Also, examples W and X contain nitrogen, which carboxylic acids do not contain. 4. 4 Esters Func. Groups-2 H H These are not esters These are esters C H H O O H O H C C H O C H Y Z C H O AB AA O H O H H O H C H H O H O O AC C C H C H AD O H H N C O O H H O AE AF Circle or highlight the sequence characteristic of an ester in each structure above, define the sequence in words below (your claim), cite the specific data used to develop your claim and provide an appropriate warrant. Specific Bonding Sequence for an Ester Draw Two New Ester Structures below Esters have a carbon single-bonded to an oxygen, which is single-bonded To another carbon, which is double-bonded to an oxygen. Examples Y-AB prove this. Example AD contains a nitrogen, Which esters do not have. Example AC has the oxygen containing The single-bond attached to a hydrogen, which does not hold true For esters. 5. Amides These are amides H H N H AG C H AH O H O H C C O H C H H These are not amides H O H N C H N H H N AJ O AK H ALH C H O H N C H O H C C H AI C H H H O H H H N H AM AN H Circle or highlight the sequence characteristic of an amide in each structure above, define the sequence in words below (your claim), cite the specific data used to develop your claim and provide an appropriate warrant. Specific Bonding Sequence for an Amide Draw Two New Amide Structures below Amides have a nitrogen single-bonded to at least one hydrogen, And single-bonded to a carbon, which is double-bonded to an oxygen. Func. Groups-2 5 Examples AG-AJ prove this. Example AL, a non-amide, contains a nitrogen, but the nitrogen is single-bonded to two carbons rather than one. Example AN has a nitrogen bonded to hydrogen, and bonded to a carbon, but it’s not bonded to another carbon, which is then bonded to another carbon, rather than an oxygen. 6 Func. Groups-2 Application: Recognition of Specific Organic Functional Groups: 1. Monofunctional compounds: a. Each of the following molecules has only one functional group. Classify each according to the functional group it contains: a. c. H C H H H b. d. H H O H O H C C C H H f. O g. H C C H H C H C O O j. H C O O H H C H 2. Write a complete structural formula, other than those given elsewhere in this activity, for: a. a carboxylic acid b. an aldehyde c. an ester H H i. N O H C H H O H O C O H H H H C C H C C H O H H C C N O h. O H H H e. H C C H H H C H d. an amide H e. a ketone 3. Polyfunctional compounds: As indicated in the Functional Groups-1 activity, molecules can contain more than one functional group. Those shown below are examples of “poly functional” molecules. Identify all functional groups in the following molecules. General rules for identifying functional groups in polyfunctional molecules. a. Any heteroatom (an atom other than C or H) can be part of only 1 functional group in any molecule. b. When there seem to be two possible choices for assigning a heteroatom to a functional group, the more complex functional group is chosen. alcohol and ketone e.g. O or O H carboxylic acid CH3 O a. N H b. O O O O O N H c. O H O O d. O O O H O H e. CH3 H H O Testosterone O H Func. Groups-2 4. 7 Esters and Ethers are similar in both structure and functional group name. Collaborate as a group to write a brief paragraph of grammatically correct English sentences explaining the similarities and differences in the structures of ethers and esters. Be sure to indicate how one can distinguish between these two functional groups. Reflector’s Report Discussion: As a group, identify the major content points of this activity. What questions still remain? Strategy Analyst’s Report Discussion: As a group, identify the major things you learned in terms of the process you experienced. Functional Groups 2: Out of Class Applications A. Reading: CGWW pp. 31-37 B. Activities: 1. For each of the following compounds, circle and identify functional groups. O H N O (a.) H H O O (b.) N H (c.)H2N O Alanyl valine (a dipeptide) O O O H O (d.) H O (e.) N H Proline CH 3 O Cortisone CH 3 O H O H 8 Func. Groups-2 2. For each of the following, write a complete structural formula other than those given in either Functional Group Activity for a compound that is: a. a carboxylic acid and an alkyne b. an alkene and an amide c. an ether and an aldehyde c. a ketone and an alcohol e. an ester and an amine 3. Amines and Amides are similar in both structure and functional group name. a. Using grammatically correct English sentences, write a brief paragraph explaining the similarities and differences in the structures of the two types of compounds. Be sure to indicate how one can distinguish between the two functional groups. b. How does the relationship between amines and amides compare with that between ethers and esters, which you determined in the Functional groups-2 activity in class? 4. Which two of the following compounds would you expect to have the most similar chemical reactivity? Explain your reasoning. O O H O O H O H Applicable problems from CGWW: CHAPTER 2 - Problems: 1, 2 & 3 O O O O O O H