211-13ClDisc
advertisement

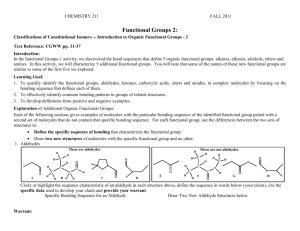
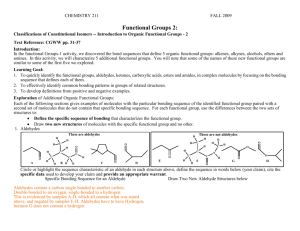
CHEMISTRY 211 FALL 2013 Functional Groups 2: SUMMARY OF CLASS DISCUSSION Classifications of Constitutional Isomers -- Introduction to Organic Functional Groups - 2 Learning Goal: 1. To quickly identify the functional groups, aldehydes, ketones, carboxylic acids, esters and amides, in complex molecules by focusing on the bonding sequence that defines each of them. 2. To effectively identify common bonding patterns in groups of related structures. 3. To develop definitions from positive and negative examples. Exploration of Additional Organic Functional Groups: Each of the following sections gives examples of molecules with the particular bonding sequence of the identified functional group paired with a second set of molecules that do not contain that specific bonding sequence. For each functional group, use the differences between the two sets of structures to: Define the specific sequence of bonding that characterizes the functional group. Draw two new structures of molecules with the specific functional group and no other. 1. Aldehydes These are aldehydes H H H C O A C B H H C O C D E C C O H H H H H C C H H C O C H H O H H These are not aldehydes H H O N C H O H F O H O G H O Circle or highlight the sequence characteristic of an aldehyde in each structure above, define the sequence in words below (your claim), cite the specific data used to develop your claim and provide an appropriate warrant. Specific Bonding Sequence for an Aldehyde Draw Two New Aldehyde Structures below Data: As indicated by the circles above, all of the structures identified Many are possible: O as aldehydes (A-D) contain double bond between a carbon and an oxygen (a carbonyl group) with the carbon atom also bonded to a carbon atom and a hydrogen atom. (E-H) do not have this complete bonding sequence. O Warrant: Because all structures identified as aldehydes contain a specific structure that is missing from all non-aldehydes provided, we can use that structure as the defining characteristic of aldehydes. Claim: The bonding sequence required for an aldehyde is a double bond between a carbon and an oxygen (a carbonyl group) with the carbon atom also bonded to a carbon atom and a hydrogen atom. 2 2. Ketones Func. Groups-2 These are ketones H H H C H H H C C O I C C J O H M K O L C H C Cl H N H C C C H H O H C C O H H H H H O H H H H O H C H These are not ketones H O H O N C H H P O Circle or highlight the sequence characteristic of a ketone in each structure above, define the sequence in words below (your claim), cite the specific data used to develop your claim and provide an appropriate warrant. Specific Bonding Sequence for a Ketone Draw Two New Ketone Structures below Data: As indicated by the circles above, all of the structures identified Many are possible: O as ketones (I-L) contain a carbonyl group with the carbon atom also bonded to two carbon atoms. (M-P) do not have this complete bonding sequence. Warrant: Because all structures identified as ketones contain a specific O structure that is missing from all non-ketones provided, we can use that structure as the defining characteristic of ketones. Claim: The bonding sequence required for a ketone is a carbonyl group with the carbon atom also bonded to two carbon atoms. In class discussion we noted that the structures of aldehydes are very similar those of ketones. They differ in the requirement for at least one hydrogen atom to be attached to the carbonyl carbon atom in aldehydes while ketones have two carbon atoms attached to the carbonyl carbon atom. Func. Groups-2 3. Carboxylic Acids 3 These are not carboxylic acids These are carboxylic acids H H H H C H H R H O C C H C H H O Q O H C O H H H O O H O H O O O C H C Br C T U H V O H H N N H H S O C H W O X O H Circle or highlight the sequence characteristic of a carboxylic acid in each structure above, define the sequence in words below (your claim), cite the specific data used to develop your claim and provide an appropriate warrant. Specific Bonding Sequence for a Carboxylic Acid Draw Two New Carboxylic Acid Structures below Data: As indicated by the circles above, all of the structures identified as carboxylic acids (Q-T) contain a carbonyl group with the carbon atom also bonded to an oxygen atom, which is also bonded to a hydrogen atom. (U-X) do not have this complete bonding sequence. Warrant: Because all structures identified as carboxylic acids contain a specific structure that is missing from all non-carboxylic acids provided, we can use that structure as the defining characteristic of carboxylic acids. Claim: The bonding sequence required for a carboxylic acid is a carbonyl group with the carbon atom also bonded to an oxygen atom which is also bonded to a hydrogen atom. Many are possible: H O H O O O In class discussion we noted that the structures of carboxylic acid and alcohols are similar. They both contain an O-H group, but the carboxylic acid has a carbonyl group attached to the O-H while alcohols do not. 4 4. Esters H H Func. Groups-2 H These are not esters These are esters H C O H O H C C C C H O Y H Z O H O H H C H O H AA O AB O H H O H O O C C C H N C H AC H AD O H O H O O H AE AF Circle or highlight the sequence characteristic of an ester in each structure above, define the sequence in words below (your claim), cite the specific data used to develop your claim and provide an appropriate warrant. Specific Bonding Sequence for an Ester Draw Two New Ester Structures below Data: As indicated by the circles above, all of the structures identified Many are possible: O as esters (Y-AB) contain a carbonyl group with the carbon atom also O bonded to an oxygen atom, which is also bonded to a carbon atom. (AC-AF) do not have this complete bonding sequence. O Warrant: Because all structures identified as esters contain a specific O structure that is missing from all non- esters provided, we can use that structure as the defining characteristic of esters. Claim: The bonding sequence required for esters is a carbonyl group with the carbon atom also bonded to an oxygen atom, which is also bonded to a carbon atom. In class discussion we noted that the structures of esters and ethers are similar. They both contain a C-O-C bond sequence, but esters have a carbonyl group attached to the oxygen atom. Func. Groups-2 5. Amides 5 These are not amides These are amides H H H C H N H O C O H N C H AG H AH O H O H C C O H N H H C H O AJ AK H O C C H H O H H N AI H C H H ALH C H H H N H N H H AM AN Circle or highlight the sequence characteristic of an amide in each structure above, define the sequence in words below (your claim), cite the specific data used to develop your claim and provide an appropriate warrant. Specific Bonding Sequence for an Amide Draw Two New Amide Structures below Data: As indicated by the circles above, all of the structures Many are possible: H identified as amides (AG-AJ) contain a carbonyl group with the carbon atom also bonded to a nitrogen atom, which is also bonded O to carbon and/or hydrogen atoms. (AK-AN) do not have this N complete bonding sequence. Warrant: Because all structures identified as amides contain a N specific structure that is missing from all non- amides provided, O we can use that structure as the defining characteristic of esters. H Claim: The bonding sequence required for amides is a carbonyl group with the carbon atom also bonded to a nitrogen atom, which is also bonded to carbon and/or hydrogen atoms. In class discussion we noted that the structures of amines and amides are similar. They both contain a nitrogen atom, but amides have a carbonyl group attached to the nitrogen atom. 6. Relationships among functional groups: a. Which functional groups have no heteroatoms? How are they differentiated? Provide your warrant. Alkenes Alkynes Contain a C=C double bond Contain a CC triple bond b. Which functional groups have 1 oxygen atom and no other heteroatoms? How are they differentiated? Provide your warrant. only single bonds -> Alcohols Contain an O attached to an H and a saturated carbon atom C=O bond Aldehydes C=O carbon attached to an H and a carbon Ethers Contain an O attached to 2 carbon atoms Ketones C=O carbon attached to two carbons 6 Func. Groups-2 c. Which functional groups have 2 oxygen atoms? How are they differentiated? Provide your warrant. Both have C=O bonded to O and carbon Carboxylic acids C=O O bonded to OH and a carbon Esters C=O bonded to O-C and C d. Which functional groups have 1 nitrogen atom and no other heteroatoms? How are they differentiated? Provide your warrant. Amines Amides Nitriles (From Exam 1 Questions) Amines have N single bonded to at least one carbon and only to Carbon or Hydrogen (note similarity to Amide – but no C=O) Amides have N attached to a C=O carbon and only to Carbon or Hydrogen (note similarity to Amine – but with C=O) Nitriles have an N that is triple bonded to a Carbon. e. What structural characteristic is common to all functional groups in this activity and missing in those explored in functional groups-1? All contain a C=O group As noted in the class discussion, this is one way to organize the functional groups to aid in remembering them. There are several other relationships that could be used and individual students should determine the organization that works best them. Func. Groups-2 7 Application: Recognition of Specific Organic Functional Groups: 1. Monofunctional compounds: a. Each of the following molecules has only one functional group. Classify each according to the functional group it contains: carboxylic acid a. c. H H C H O O H C C N C C H b. d. H H H O H O C C H H ester carboxylic acid i. C H H O j. H H C C O amide aldehyde H C O C H C H H H H ester 2. Write a complete structural formula, other than those given elsewhere in this activity, for: a carboxylic acid O .an aldehyded. O b an amide c. an ester O O N H e. a ketone O H O O H ketone O N C C O g. H H C H O C C H H H C H H O H H O C aldehyde O H H O f. C H amideH h. H H H H e. ketone H 8 Func. Groups-2 3. Polyfunctional compounds: General rules for identifying functional groups in polyfunctional molecules. a. Any heteroatom (an atom other than C or H) can be part of only 1 functional group in any molecule. b. When there seem to be two possible choices for assigning a heteroatom to a functional group, the more complex functional group is alcohol and ketone e.g. O or H chosen. O ester Ketone O a. N H 4. O O b. O carboxylic acid Amide O N Amine c. O H O carboxylic acid Ketone Ether Ether O d. O O Ketone CH3 Ocarboxylic acid CH3 H e. O H O Alcohol H O alcohol H Ketone O alkene Testosterone Esters and Ethers are similar in both structure and functional group name. Collaborate as a group to write a brief paragraph of grammatically correct English sentences explaining the similarities and differences in the structures of ethers and esters. Be sure to indicate how one can distinguish between these two functional groups. Esters and ethers both have a bonding sequence C-O-C. The difference is in the structure around on of the carbons attached to the oxygen. In the amide one of the carbon atoms attached to the oxygen atom is a carbonyl carbon while in ethers the carbon atoms attached to O are bonded only to C or H atoms. Reflector’s Report Discussion: As a group, identify the major content points of this activity. Ability to recognize the bond sequences of carbonyl group containing functional groups in complex molecules. What questions still remain? Strategy Analyst’s Report Discussion: As a group, identify the major things you learned in terms of the process you experienced. Experience with recognizing common structures in relatively complex molecules. Experience with writing clear definitions of bonding sequences that identify a particular class of molecules. Experience with organizing classifications to focus on similarities and differences to assist in relating structures to names. H