1.4-CO
advertisement

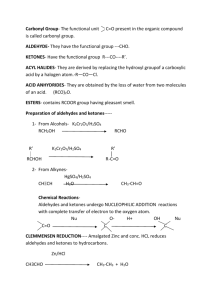
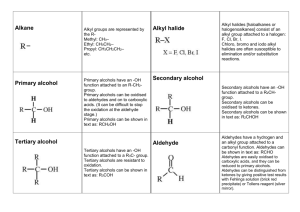
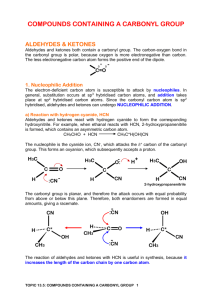
1.4 Functional Groups with C = O Bond C=O is called a carbonyl group Aldehydes organic compounds with the carbonyl group(C=O) on the end of a chain general formula Example Ketone organic compound that has a double bonded oxygen on any carbon within the carbon chain General formula Example Naming aldehydes 1. Name the parent alkane. label carbons from side closest to the functional group 2. Replace –e with –al. No need to give a position number for the carbonyl group because it will always be on carbon 1 Naming Ketones 1. 2. 3. 4. Name the parent alkane main chain must contain the C=O group If only one ketone group replace –e with –one. if more than one ketone group keep the –e suffix and a suffix such as –dione or –trione Position numbers are needed for carbon chains more than 4 carbons long. # carbon chain so that the functional group is on the lowest possible Carbon Physical properties of aldehydes and ketones C=O is polar so aldehydes and ketones are polar Shorter the chain more soluble and more polar. Less soluble if longer and less polar boiling points of aldehydes and ketones are lower than corresponding alcohols and higher than corresponding alkanes Additional Characteristics of aldehydes and ketones strong pungent smell for aldehydes, ketones sweet smell longer the aldehyde more pleasant smelling ex cinnamaldehyde often in perfumes since they are polar can act as polar solvents, because of the non-polar hydrocarbon side they can also act as non-polar solvent (Demo) Carboxylic acids organic compound with a Carboxyl group (COOH General formula examples Naming a carboxylic acid 1. Name the parent alkane 2. replace the –e with –oic acid 3. Label the chain from side closest to the functional group. carboxyl group is always on Carbon 1 in a carboxylic acid Physical Properties of Carboxylic acid Polar Smaller chains are more soluble and solubility decreases as chain size increases. melting points and boiling points are very high Additional Characteristics often unpleasant odours the –OH group does not behave like the basic Hydroxide ion (OH-) Carboxylic derivatives Ester General formula Naming Esters (Names are made in 2 parts) 1. Identify the main part of the ester which contains the C=O group. Name the parent acid 2. Replace –oic acid with –oate 3. The second part of the name is the alkyl group that is attached to the -O atom. 4. put names together Physical Properties usually polar small esters soluble low boiling points and volatile at room temp Additional Characteristics pleasant odours and tastes, perfumes and artificial flavours Amides Combination of a carboxylic acid and ammonia or an amine carbon atom double bonded to an oxygen and single bonded to a nitrogen general formula Examples Naming an amide 1. Name the parent carboxylic acid (side that contains C=O) C=O side always position 1 2. replace –oic acid with suffix –amide 3. Decide if a. primary i. If there are 2 Hydrogen atoms and no alkyl groups attached to the Nitrogen. ii. No prefixes iii. b. secondary i. if there is one alkyl group attached to the nitrogen (N) ii. name the alkyl group and give it location letter N to indicate that it is bonded to the Nitrogen iii. c. tertiary i. if there are 2 alkyl groups ii. place alkyl groups in alphabetical order iii. use location Letter N- before each group to indicate it is bonded to Nitrogen iv. if groups are identical use N,N- v. 4. Put name together: Prefix + Root + Suffix. Physical Properties of Amides similar to carboxylic acids soluble in water decreases with size Primary amides much higher melting points and boiling points than carboxylic acids many simple amides are solids at room temp Additional Characteristics acetaminophen pain killer Urea in urine of many mammals used as fertilizer.