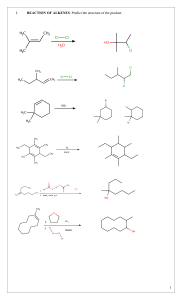
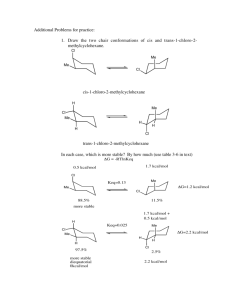
Alkynes\Why are Alkenes more reactive than Alkynes
advertisement

CHEM 109A CLAS Why is an Alkyne Less Reactive Than and Alkene? When alkenes react with electrophiles, a carbocation is formed. We learned that carbocations are stabilized by hyperconjugation – the more hyperconjugation the more stable 3o > 2o > 1o > Me When alkynes react with electrophiles, a vinylic carbocation is formed (where the + charge is on a vinyl/sp hybridized C). Vinylic carbocations are LESS stable than previous carbocations (where the + charge is on an sp2 hybridized C) b/c the geometry does not allow for effective hyperconjugation. Putting them in order of decreasing stability (pg. 266)… The less stable the intermediate, the higher the activation energy barrier for the reaction and therefore the slower the reaction. Figure 6.2 from Bruice 5th Ed. Page 1 of 2 CHEM 109A CLAS Why is an Alkyne Less Reactive Than and Alkene? If we take the example reactions of ethane and ethyne/acetylene with HBr and look at the first step… C2H4 + HBr → H3C-C+H2 + BrBDE: 174 87 101 kcal/mol (From Table 3.2, pg146) 1o C+ ΔH = 621-101 = 160 kcal/mol ΔG* ~ 160 kcal/mol C2H2 + HBr → H2C-C+H + BrBDE: 231 87 ~109.5 1o vinylic C+ ΔH = 318-109.5 = 208.5 kcal/mol ΔG* ~ 208.5 kcal/mol kcal/mol (From Table 3.2, pg146) *ΔS for both reactions is ~ 0 (2 reactants form 2 products so no loss of “freedom” or “disorder”) Both reactions are endergonic, so the T.S.s are similar in energy and structure to the products (Hammond Postulate). The product of the alkyne reaction (a 1o vinylic C+) is less stable than the product of the alkene reaction (1o C+) so the TS for the alkyne reaction must be less stable/higher in energy than the TS for the alkene reaction (EvansPolanyi Principle). Alkyne reaction therefore has a higher activation energy barrier (see Fig 6.2 above) and will proceed slower than the alkene reaction. Page 2 of 2