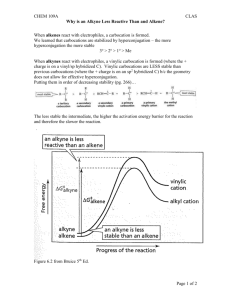
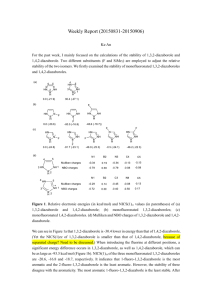
Supplemental Information
advertisement

Supplemental Information 1. Figure S1: Snapshot of a single C18 ligand capped nanocrystal. Size of the nanocrystal is 4 nm. Coverage density is about 4 ligands/nm2. a) b) c) d) 2. Figure S2: Snapshots of 3 nm NC with different ligand coverage densities. a) 2.5 ligands/nm2, b) 3 ligands/nm2, c) 3.5 ligands/nm2 and d) 4 ligands/nm2. 3. Figure S3: Graph of Interaction Energy Curve vs ligand capping density for a 3.1 nm Nanocrystal. 4. Figure S4: Graph of Interaction Energy Curve vs ligand length for a 3.1 nm Nanocrystal. 5. Figure S5: Differential scanning calorimetry of 7 nm nanocrystal capped with C18 oleic acid ligands with a coverage of about 3.0 ligands/nm2. The various curves are for different processing solvents in the lattice. A peak in the curves would indicate a phase transition such as melting or boiling. The only peak seen is for toluene at around 100 ˚C. This corresponds to evaporation of residual toluene in the superlattice. If there were ligand bundling, and the bundles underwent a melting transition from bundled to un-bundled state, there would be a peak observed in the specific heat capacity curve with temperature. No such peak is seen until 120 ˚C. It is therefore, unlikely that ligands are strongly bundled. (The small dips that are seen around 60 ˚C are due to some trapped hexane in the lattice since all the NC samples were initially dissolved in hexane and then processed in other solvents.) 6. Bond CH2-CH2 CH2-CH3 Angle CH2-CH2-CH2 CH2-CH2-CH3 Dihedral CH2-CH2-C2-CH2 CH2-CH2-C2-CH3 Non-bonded Interactions CH2 CH3 r0 (Å) 1.54 1.54 θ0 (˚) 109.5 109.5 K1 (kcal/mol) 1.412 1.412 σ (Å) 3.90 3.90 K [kcal/(mol/Å2)] 260 260 K´ [kcal/(mol/deg2)] 63 63 K2 (kcal/mol) -0.272 -0.272 ε (kcal/mol) 0.12 0.175 Table I Force Field Parameters for the Jorgensen United Atom Model [1]. K3 (kcal/mol) 3.15 3.14 7. Bond CH2-CH2 CH2-CH3 Angle CH2-CH2-CH2 CH2-CH2-CH3 Dihedral CH2-CH2-C2-CH2 CH2-CH2-C2-CH3 Non-bonded Interactions CH2 CH3 r0 (Å) 1.53 1.53 θ0 (rad) 1.92 1.92 k1 (kcal/mol) 1.6 1.6 σ (Å) 4.009 4.009 k [kcal/(mol/Å2)] 634 634 k´ [kcal/(mol/deg2)] 120 120 k2 (kcal/mol) -0.867 -0.867 ε (kcal/mol) 0.09344 0.22644 k3 (kcal/mol) 3.24 3.24 Table II Force Field parameters for the Paul et al. United Atom Model [2]. References: [1] W. L. Jorgensen, J. D. Madura and C. J. Swenson, J. Am. Chem. Soc., 106, 6638-6646 (1984) [2] W. Paul, Do Y. Yoon and G.D. Smith, J. Chem. Phys. (1995) 103, 1702-1709