Ch. 8 & 9 Topics and Tips
advertisement

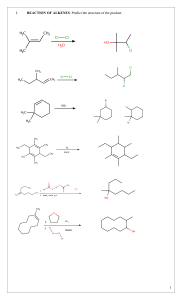
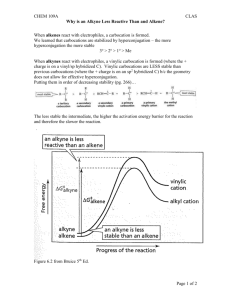
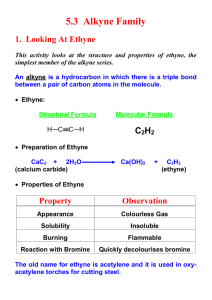
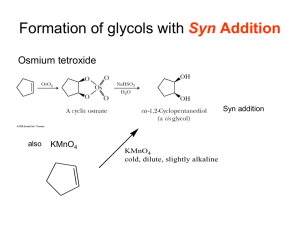
Leader: Hannah J. Course: Chem 331 Supplemental Instruction Instructor: Dr. Winter Iowa State University Date: 11/1/15 The following is a (checkable) list of the topics that you should know and understand from chapters 8 & 9: Be able to give the missing starting materials, reactant(s), and product(s) given the other two for all reactions covered in lecture. o Most of these reactions are included in the alkene and alkyne summary sheets posted on Blackboard Learn and in the summary of reactions at the end of each chapter in the textbook o See the reaction tree summary done in class 10/30/15 Ch. 8 & 9 Topics Be comfortable providing a mechanism including arrow pushing and intermediates for all reaction mechanisms covered in lecture. There will be at least one (probably multiple) of these on the exam. This includes: o Alkenes Br2 or NBS Br2 or NBS and H2O HBr H2 and Pd/C CHCl3 and NaOH Markovnikov addition of water (with 1) Hg(OAc)2, H2O 2) NaBH4) Reaction of an epoxide with H3O+ o Alkynes Br2 or NBS HBr H2 and Pd/C NaNH2 and R-CH2-X (where R is primary or methyl and X is Br or I) Tautomerization (rearrangement) of an enol to a ketone within the Markovnikov or anti-Markovnikov addition of water (hydration) o Check your notes on these! This is what I have based on lecture 10/30/15, but there may be a couple that I have missed. o Don’t forget about possible carbocation rearrangements (hydride shift, alkyl shift, and ring expansion) o Don’t be thrown if you see a reaction on the exam that looks a little different from what we’ve done. It will be similar to a reaction or two that we’ve covered, so you should be able to do it if you understand the principle/logic behind the mechanisms we’ve covered. Be comfortable doing synthesis problems: that is, be able to give a multi-step pathway (no mechanisms required) to reach a more complicated product from a simpler starting material. One good way of doing these problems is by retrosynthetic analysis; in other words, by working backwards. This will take some practice! There will be one of these on exam 3. o Make sure that you have a good understanding of the reaction to form and use an acetylide ion to add Carbons onto an alkyne. This is the only reaction we’ve covered to add Carbons onto a chain, so there is a good chance that it will show up on the exam. Note that you could start with either the alkyne/acetylide ion or the R-CH2-X and add the other to form a longer carbon chain. 1060 Hixson-Lied Student Success Center 515-294-6624 sistaff@iastate.edu http://www.si.iastate.edu o Use problems from Sapling, the textbook, SI worksheets, etc. Pay attention to the “twists” in alkyne reactions as compared to alkyne reactions. Many alkyne reactions are just alkene reactions done twice, but a few look a little different. For example, alkyne oxidative cleavage and hydration (Markovnikov or anti-Markovnikov addition of water) reactions give slightly different products and/or use slightly different reagents than their alkene counterparts.