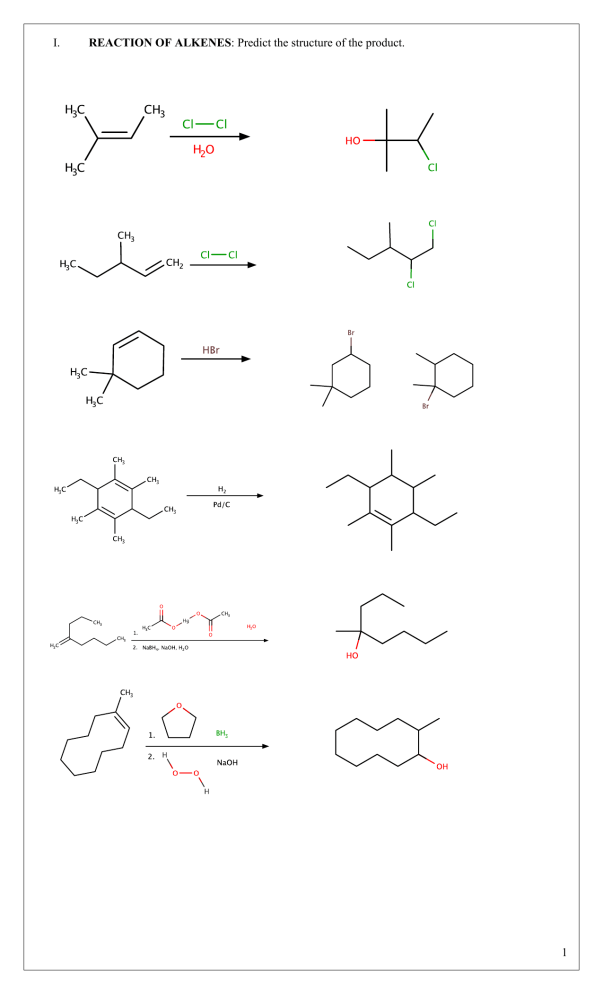
I. REACTION OF ALKENES: Predict the structure of the product. 1 2 II. REACTION OF ALKYNES: Predict the structure of the product. 3 4 III. ORGANIC SYNTHESIS A. Illustrate the reaction and identify the reagent used in the following synthesis: 1. 1,2-dimethylcyclohexane from 1,2-dimethylcyclohexa-1,3-diene 2. trans-1,2-diphenylacetylene (byproduct: sodium iodide and ammonia) from ethynylbenzene 3. 3-ethyloctan-2-ol from 3-ethyloct-2-ene. 4. 3-methylpentane-1,5-dioic acid from 3-methylcyclopentene 5. (Z)-hex-3-ene from hex-3-yne 6. But-2-yne from 2,3-dibromobutane 5 7. Hexa-2,3-diol from hex-2-ene 8. 3-methylbutanoic acid (byproduct: carbon dioxide) from 4-methylpentyne 9. A racemic mixture of trans-1-ethyl-2-iodocyclopentanol from 1-ethylcyclopentene 10. 2,2,3,3,5,5,6,6-octachloroheptane from hepta-2,5-diyne B. Predict the structure and the IUPAC name of the substrate/s if it was an: 1. Alkyne 6 Butyne 2. Alkane (four possible alkanes) (1-halopropyl)cyclopentane or 1halo-1-cyclopentylpropane (2-halopropyl)cyclopentane or 2halo-1-cyclopentylpropane (1-hydroxypropyl)cyclopentane or 1-cyclopentylpropanol (2-hydroxypropyl)cyclopentane or 1-cyclopentylpropan-2-ol or 1- cyclopentylisopropanol 3. Alkyne 1-(prop-1-ynyl)cyclopentane or 1-cyclopentylpropyne 4. Alkene Cyclooctene 5. Alkyne 7 Cyclooctyne 6. Alkene Hept-2-yne 7. Alkene Pent-2-yne 8. Alkene Diphenylethylene 9. Alkene 1,2-dichloro-3,4,5trimethylheptene 10. Alkyne 8 3,4,5-trimethylheptyne 11. Alkane 1-isopropyl-2-methylcyclopentane 12. Alkenylated Alkane 1-methyl-2-(propan-2ylidene)cyclopentane 13. Alkene 5-isopropyl-2-methylhept-3-ene or 9 5-ethyl-2,6-dimethylhept-3-ene 14. Alkyne 5-isopropyl-2-methylhept-3-yne or 5-ethyl-2,6-dimethylhept-3-yne 15. Alkene 6-ethyl-5-propylundec-5-ene 16. Alkyne Oct-3-yne C. Complete the following synthesis by indicating the missing reagent and/or drawing the structure of the missing substrate and/or product. i. ii. 10 iii. iv. 11 v. 12