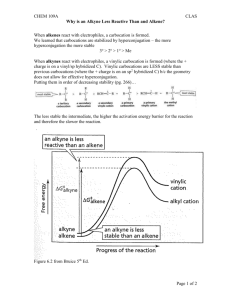
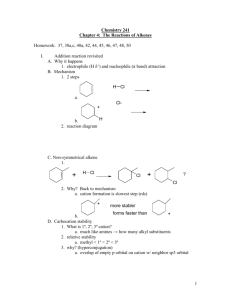
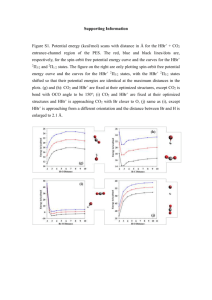
Unit 3: Reactions of Alkenes. Thermodynamics and Kinetics
advertisement

Unit 3: Reactions of Alkenes. Thermodynamics and Kinetics Hydrocarbons that contain only C-C bond are called alkanes Hydrocarbons that contain C=C bond are called alkenes or olefins (oil forming) 尤加利樹油 3.1 Molecular Formula and the Degree of Unsaturation Alkane Alkene CH3(CH2)nCH3 CH3(CH2)nCH3 CnH2n+2 Cyclic alkane CnH2n CnH2n Cyclic alkene CnH2n-2 H C H 2 (C H 2 ) n H 2C H (CH 2 ) n Degree of unsaturation = 2 1 p bond or 1 ring, degree of unsaturation = 1 3.3 The Structure of Alkenes 3.4 cis-trans Isomerism Rotational barrier 63kcal/mol H3C—CH3 rotational barrier = 2.9 kcal/mol Cis-Trans Interconversion in Vision cis-trans Isomerism 3.6 Reactivity Considerations Functional group Organic Reactions • Electron-rich atoms or molecules are attracted to electron-deficient atoms or molecules • Nucleophile: an electron-rich atom or molecule • Electrophile: an electron-deficient atom or molecule • A nucleophile and an electrophile react with each other Electrophiles and Nucleophiles ` Mechanism of the Reaction 3.7 Thermodynamics and Kinetics Reaction coordinate digram Thermodynamics Describes the properties of a system at equilibrium The more stable the compound, the greater its concentration at equilibrium Gibbs standard free energy change This symbol indicates that the reaction takes place under standard conditions --all species at 1 M, 25 OC, and 1 atm. ↓ R is the gas constant (1.986 cal/mol OK) T is the absolute temperature (OK) Free Energy, Enthalpy and Entropy DHO < 0, exothermic reaction; DHO > 0, endothermic reaction In condensed phase, DSO ≈ 0. Therefore DGO ≈ DHO Calculate DHO for a Certain Reaction p.130 Solvation: the interaction between a solvent and a molecule (or ion) in solution Solvation can have a large effect on the DHO of a reaction, and it can also affect the DSO of a reaction. Kinetics Deals with the rates of chemical reactions and the factors that affect those rates Free energy of activation Rate Law First-order reaction Second-order reaction Second-order reaction The Arrhenius equation: Rate Constant and Equilibrium Constant k1 A B k -1 At equlibrium, forward rate = reverse rate. k1 [A] = k-1 [B] therefore [B] k1 K eq = = k -1 [A] Reaction Coordinate Diagram for the Addition of HBr to 2-Butene Bonds being broken Bonds being formed p DH = 61 kcal/mol C-H DH = 101 kcal/mol H-Br DH = 87 kcal/mol DHtotal = 148 kcal/mol Total DH change = +47 kcal/mol Bonds being formed C-Br DH = 69 kcal/mol Over all DH change = -22 kcal/mol Reaction Coordinate Diagram for the Addition of HBr to 2-Butene -22 kcal/mol 3.8 General Mechanism for Electrophilic Addition 3.9 Addition of Hydrogen Halides H 2C CH 2 + HCl CH 3 CH 2 Cl Relative Stabilities of Carbocations sp3 Inductive effect sp2 Relative Stabilities of Carbocations 3.11 The Structure of the Transition State The Hammond postulate 3.12 Regioselectivity of Electrophilic Addition Reactions Constitutional isomers Major product Major product Minor product Minor product Regioselective reaction Br Br H 3 C HC CH CH 2 CH 3 + HBr H 3 C CH CH 2 CH 2 CH 3 + H 3 C H 2 C CH CH 2 CH 3 Non-regioselective reaction H 3 C H 2 C CH CH2 H vs H 3 C H 2 C HC H CH2 3.13 Addition of Water and Alcohols hydration Addition of Alcohol to Alkene 3.14 Rearrangement of Carbocations According Markovnikov’ rule This compound should be major product Mechanism for the Formation of Rearranged Product a secondary carbocation attack on unrearranged carbocation minor product a tertiary carbocation attack on rearranged carbocation major product Mechanism for the Formation of Rearranged Product a secondary carbocation attack on unrearranged carbocation minor product a tertiary carbocation attack on rearranged carbocation major product Carbocation rearrangements also can occur by ring expansion 1,2-alkyl shift 3.15 Addition of Halogens p.154 last sentence unstable F2 reacts explosively with alkenes!!! Formation of Halohydrins 3.18 Addition of Radicals. The Relative Stabilities of Radicals Radical addition reaction Addition of HBr through Radical Mechanism Addition of HBr through Radical Mechanism 3.19 Addition of Hydrogen. The Relative Stabilities of Alkenes Heat of Hydrogenation Relative Stabilities of Alkenes