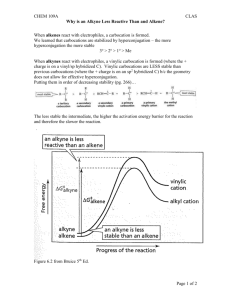
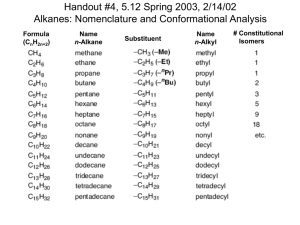
Additional Problems for practice: 1. Draw the two chair
advertisement

Additional Problems for practice: 1. Draw the two chair conformations of cis and trans-1-chloro-2methylcyclohexane. Cl Me Me Cl cis-1-chloro-2-methylcyclohexane H Me Cl Me H H H Cl trans-1-chloro-2-methylcyclohexane In each case, which is more stable? By how much (use table 3-6 in text) ΔG = -RTlnKeq 1.7 kcal/mol 0.5 kcal/mol Cl Me Keq=0.13 ΔG=1.2 kcal/mol Me Cl 88.5% 11.5% more stable 1.7 kcal/mol + 0.5 kcal/mol H Keq=0.025 Me Cl Me ΔG=2.2 kcal/mol H H H 97.5% more stable diequatorial 0kcal/mol Cl 2.5% 2.2 kcal/mol 2. Which isomer is more stable, cis or trans decalin? Explain your answer in terms of the steric interactions of the two molecules. H No 1,3 diaxial interactions H trans-decalin (rigid) H H H H H H H H cis-decalin cis-decalin extensive 1,3-diaxial interactions Thus, trans-decalin is more stable than cis-decalin 3. Draw the following disubstituted cyclohexanes and label the substituents as axial or equatorial. The substituents can be any alkyl group of your choosing. Remember to include the ring-flip structure as well. (a) 1,3 trans disubstituted (b)1,3-cis disubstituted (c)1,5-cis disubstituted (d) 1,4-cis disubstituted (e) 1,5-trans disubstituted (f) 1,6-trans disubstituted ax a eq ax eq ax ax b eq eq ax eq ax c eq ax ax d eq eq ax e eq eq ax ax eq f eq ax