Bond Angle & Hybridization Worksheet: Chemistry Practice
advertisement

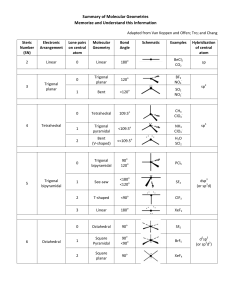
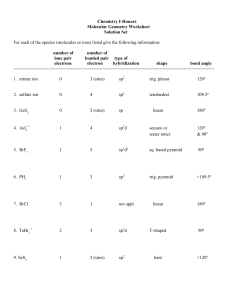
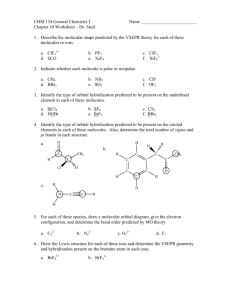
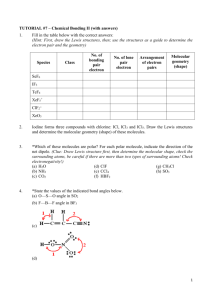
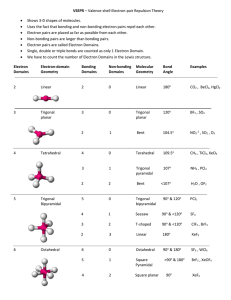
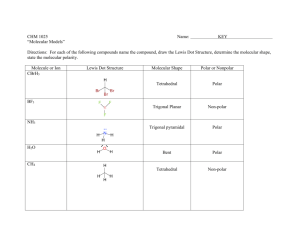
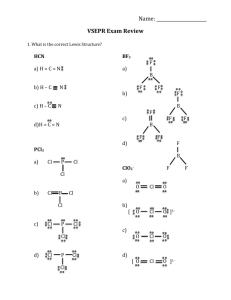
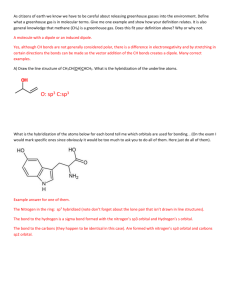
TRO 1st ed AND Chang, 9th Edition, Chapter 10, Worksheet #1 Answers: 1. In each case, predict (a) the approximate bond angle(s), (b) the hybridization around the underlined atom. (Note: It is helpful to first sketch the Lewis stucture!) Molecule or Ion (1) OF2 (2) H2CO (3) NO2+ (4) BF3 (5) SbF5 (a) No. of valence e - ‘s (b) Lewis structure 20 12 16 24 40 (c) Approximate bond angle(s) (d) Hybridization 109.5o 120o 180o 120o 90o, 120o sp3 sp2 sp sp2 sp3d (e) Polar or non-polar molecule? (f) Geometry name polar polar non-polar non-polar bent trigonal planar Ion: Not applicable linear trigonal planar trigonal bypyramidal ______________________________________________________________________________ 2. For each of the molecules below fill in the indicated items in the chart. The central atoms are underlined. Molecule (4) CH2Cl2 (1) SO2 (2) HBF2 (3) XeF4 (5) NF3 (a) No. of valence e - ‘s 18 18 36 20 26 (c) Approximate bond angle(s) (d) Hybridization 120o 120o 90o 109.5o 109.5o sp2 sp2 sp3d2 sp3 sp3 (e) Polar or non-polar molecule? (f) Geometry name polar polar non-polar polar polar bent trigonal planar square planar tetrahedral trigonal pyramidal (b) Lewis structure ____________________________________________________________________________ 3. Predict (a) the approximate bond angle, (b) the hybridization around the indicated atoms (the atoms to which the arrows are drawn in the structures below). Write your answers near the corresponding labels (1 to 5) in the drawings. (Note: the lone pairs on the F atoms are omitted.) H H N 1 F S F F F 2 F F Br 4 C N F 3 C N 5 F F C H C C N N H page 1 O H TRO 1st ed AND Chang, 9th Edition, Chapter 10, Worksheet #1 (1) 90o, 120o; sp3d (2) 90o; sp3d2 (3) 109.5o; sp3 page 2 (4) 120o; sp2 (5) 109.5o; sp3