Chemistry Bonding Exercises: Lewis Structures & Molecular Geometry
advertisement

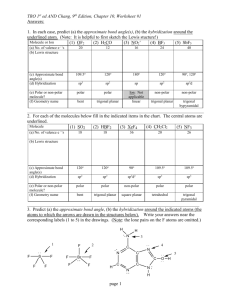
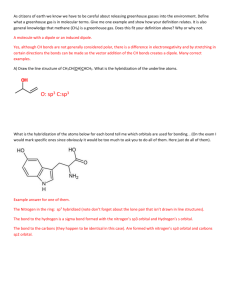
Chemistry 105, Chapter 7 Exercises Types of Bonds 1. Using the periodic table classify the bonds in the following compounds as ionic or covalent. If covalent, classify the bond as polar or not. MgBr2 CF4 H H C O H H SiO2 Ca(NO3)2 HOF Br2 H O H C C ONa H Ionic Compounds 2. Complete and balance the following combination reactions showing the formation of an ionic compound. a. Na + I2 → b. Mg + I2 → c. Fe + O2 → (for the product iron is +3) d. Ca + N2 → e. Fe + S → (for the product iron is +3) f. Rb + N2 → Lewis Structures 3. Write the Lewis structures for the following molecules and polyatomic ions. Unless otherwise noted, the first atom is the central atom. a. NH3 b. CCl4 c. BrF2+ d. COCl2 e. SCN- (C is the central atom) f. CO g. HCO+ (C is the central atom) h. CN- i. XeF2 j. IO2k. XeO2F2 l. BF3 m. SeBr6 n. ICl4o. SO42- 4. Draw Lewis structures (show all e- pairs) for the following species. The skeleton structure is shown. Multiple bonds may exist between bonded atoms. H H C C N H H H C C H H H O H C C H H H O C O H O F Cl C C F Cl H O O H C C O O 5. For the following compounds the correct number of bonds is shown. Supply the lone pairs of electrons. H H C O O H C C H H H H O N C C H H H O H H O H H C Cl H 6. Draw resonance structures for the following. NO2Cl (N is the central atom. The O’s and Cl are bonded directly to N) SO3 NO2NNO (This is also the skeleton structure.) HCO2- (C is the central atom. The O’s and the H are bonded directly to the C.) Formal Charges 7. Assign formal charges to each element in the following compounds. H O F O N O O H C O S C N H H C C H H H Br Cl Cl Cl 8. For the following two resonance structures assign formal charges and decide which is the most likely structure. N N N N N N 9. For the following two resonance structures assign formal charges and decide which is the most likely structure. You must first supply the lone pairs of electrons. H O C N H O C N Hybridization, Molecular Geometry, Molecular Polarity, Sigma and Pi Bonds 10. If the following atomic orbitals overlap, what type of hybrid orbital forms? What is the bond angle separating these? a. an s and one p orbital b. an s and three p orbitals c. an s and two p orbitals 11. For the following compounds and ions: a. write the electron dot structure b. draw the 2-D representation of the 3-D shape c. give the molecular geometry d. give the bond angle around the central atom e. indicate if the molecule is polar f. give the hybridization for each atom (except where indicated not to) ClO2SF6 (skip hybridization) CO32PCl5 (skip hybridization) BF3 SF42- (skip hybridization) SCO (C is the central atom) IBr2- (skip hybridization) NO3RnF4 (skip hybridization) NH2Cl (N is the central atom, all atoms are bonded to N) CH2Br2 (C is the central atom) SCN- 12. Show the charge distribution in the following and indicate which molecules are polar. Cl Cl Cl H H C C H H H C C Cl F C C Cl H N N Cl F N N F F 13. Give the hybridization for each atom (except H) in the following organic solvent. Unshared electrons are not shown. How many sigma and pi bonds are there? H3C C CH3 O 14. Give the hybridization for the nitrogens and oxygens in the following. Unshared electrons are not shown. How many sigma and pi bonds are there in each? O N O O H N H Cl N N N N O 15. Give the hybridization for the carbons and oxygens in the following. Unshared electrons are not shown. How many sigma and pi bonds are there in each? 2O C O H3C Cl H C OH O C O O O Chapter 7, Answers to Exercises 1. MgBr2 ionic CF4 polar covalent SiO2 polar covalent CaNO3 ionic between the Ca2+ and the NO32- but polar covalent between N and O HOF polar covalent Br2 nonpolar covalent nonpolar polar H H C O H H nonpolar polar polar H O H C C ONa H polar ionic 2. a. 2Na + I2 → 2NaI b. Mg + I2 → MgI2 c. 4Fe + 3O2 → 2Fe2O3 d. 3Ca + N2 → Ca3N2 e. 2Fe + 3S → Fe2O3 f. 6Rb + N2 → 2Rb3N 3. H N H H Cl Cl C Cl Cl O Cl C Cl F Br F S C N C O F H C O C N Xe F Br F B F F Br Br Se Br O I O F Cl Br Cl Br I Cl Cl O Xe O F O O S O O 4. H H C C N H H H C C H H H O H C C H H H O C O H O Cl F C C F Cl H O O H C C O O 2- 5. H H C O H O H C C H H H O N C C H H H O H H O H H C Cl H 6. Cl O N O Cl O N O Cl O N O O O S O O O S O O O S O O N O N N O H O C O O N O N N O N N O H O C O 7. 0 0 0 H O F 1- 0 0 O N O 1- H 0 H C C H H H 0 O 1H C O 0 0 0 0 1S C N 0 Br 0 Cl Cl 0 Cl 0 8. 2- 1+ N N 1- 0 N N 1+ N 1N most likely 9. 0 H 0 C O 0 N 1+ H O 0 C 1N most likely 10. a. an s and one p orbital: sp hybrid orbital, 180o b. an s and three p: sp3, 109.5oC c. an s and two p: sp2, 120o 11. linear, 180o, polar molecule, S sp2, C sp, O sp2 S C O Br I Br O F F - F F N Br C trigonal planar, 120o, nonpolar, N sp2, =O sp2, -O sp3 O Rn H H O N linear, 180o, nonpolar HCl square planar, 90o, nonpolar trigonal pyramid, ~109.5o, polar, N sp3, Cl sp3 tetrahedral, 109.5o, polar, C sp3, Br sp3 Br H S C linear, 180o, polar, C sp, S sp2, N sp2 N - O O F F O bent, ~109.5o, polar, Cl sp3, O sp3 Cl F S F F F octahedral, 90o, nonpolar 2- O C trigonal planar, 120o, nonpolar, C sp2, =O sp2, -O sp3 O Cl Cl P Cl Cl Cl trigonal bipyramid, 90o and 120o, nonpolar F F B trigonal planar, 120o, nonpolar, F sp3, B sp2 F 2- F F F F square planar, 90o, nonpolar 12. Cl Cl Cl C C H polar H H C C H H Cl C C Cl nonpolar H F N N Cl polar F nonpolar N N F F polar 13. Both CH3 C’s are sp3, the central C is sp2, O is sp2. There are 9 sigma bonds and 1 pi bond. 14. O N O O -O sp3 =O sp2 N sp2 3σ 1π H N H Cl N N N N O N sp3 3σ sp 2π 1σ N sp O sp3 2π 2σ 15. H3C Cl sp3 4σ O C O O sp2 C sp 2σ 2π O C O O C sp2 -O sp3 =O sp2 3σ 1π 2- H C OH O C sp2 -O sp3 =O sp2 4σ 1π