File
advertisement

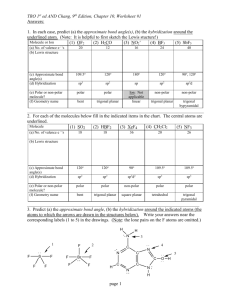
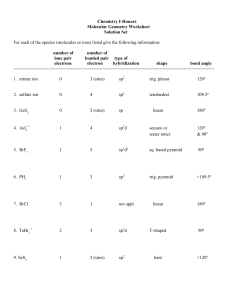
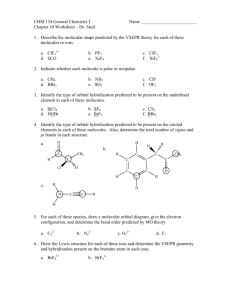
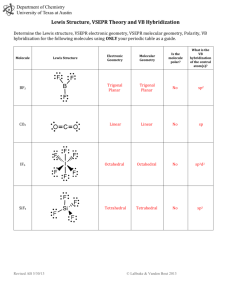
Practice Test Chapter 10 1. The electron domain geometry (base structure) of which of the following is Td? A. CBr4 B. PH3 C. CCl2Br2 D. XeF4 E. all of these except XeF4 2. The molecular geometry of CS2 is A. linear B. bent C. tetrahedral 3. The F-B-F bond angle in BF2- is A. <109.5o B. 109.5o C. 120o D. trigonal planar D. < 120o E. T-shaped E. 180o 4. Of the following species, which will have bond angles of 120o? A. PH3 B. ClF3 C. NCl3 D. BCl3 E. all of these 5. What is the shape of ClF4+? A. Tetrahedron B. Trigonal bipyramid C. Seesaw D. T-shape E. square pyramid 6. According to VSEPR, if there are 3 electron domains and 1 nonbonding pair of electrons on a central atom, they will be arranged such that the angles between the domains are ____ A. 90o B. 120o C. < 120o D. 109.5o E. <109.5o 7. The electron domain geometry (base structure) and the molecular geometry (actual shape) of an ABn molecule will always be the same if A. there are no lone pairs on the central atom B. there is more than one central atom C. n is greater than 4 D. n is less than 4 E. the octet rule is obeyed 8. Which molecule below is non polar? A. PBr3 B. NF3 C. IF3 D. BF3 9. What is the shape and polarity of PF3? A. trigonal planar, polar B. trigonal planar, non polar C. trigonal pyramid, polar D. trigonal pyramid, non polar E. tetrahedron, polar 10. What is the hybridization scheme around C in CO2? A. sp B. sp2 C. sp3 D. sp3d E. sp3d2 E. BrCl3 11. What is the hybridization in BrF5 and AsF5? A. Both sp3d B. Both sp3d2 C. sp3d2 and sp3d, respectively D. sp3d and sp3d2, respectively E. Both sp3 12. Which of the following exhibit delocalized bonding? SO SO2 SO3 SO32A. B. C. D. E. All of them do SO2, SO3, SO32SO2 and SO3 SO3 and SO32None of them 13. A triple bond consist of ____ sigma bonds and ____ pi bonds. A. 3 σ and 0 B. 0 σ and 3 C. 1 σ and 2 D. 2 σ and 1 E. 3 σ and 3 14. Electrons in ___ bonds remain localized between two atoms. Electrons in ____ bonds can become delocalized between two or more atoms. A. pi, sigma B. sigma, pi C. pi, pi D. sigma, sigma E. ionic, sigma 15. What is the hybridization scheme of S in H2S? A. sp B. sp2 C. sp3 D. sp3d E. sp3d2 ANSWERS 1. 2. 3. 4. 5. E A D D C 6. E 7. A 8. D 9. C 10. A 11. C 12. B 13. C 14. B 15. C