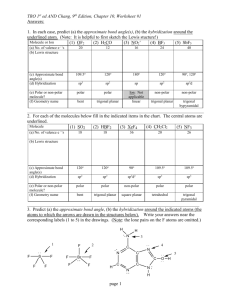
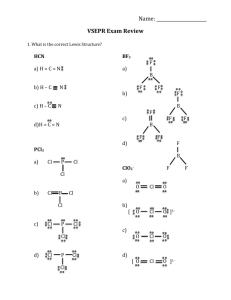
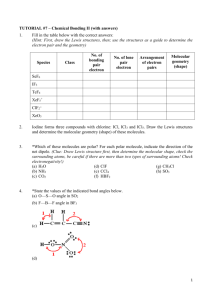
CHM 2045L
advertisement
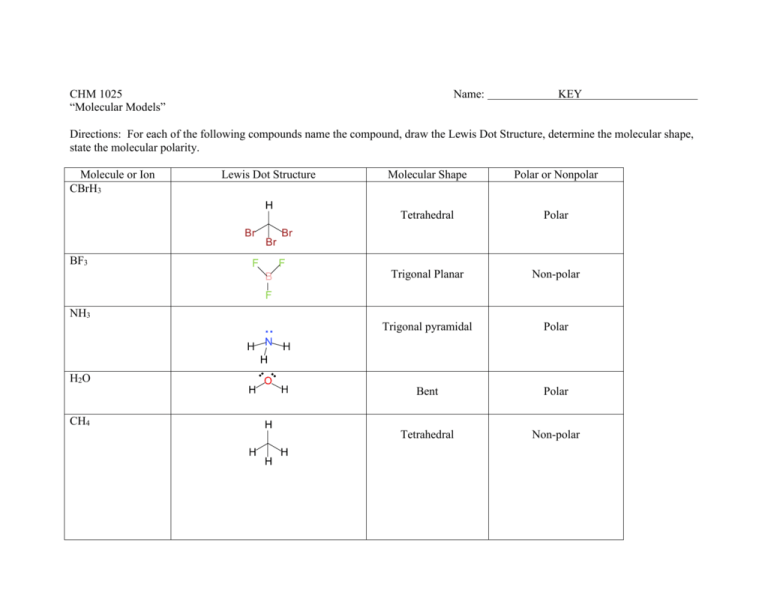
CHM 1025 “Molecular Models” Name: KEY Directions: For each of the following compounds name the compound, draw the Lewis Dot Structure, determine the molecular shape, state the molecular polarity. Molecule or Ion CBrH3 Lewis Dot Structure Molecular Shape Polar or Nonpolar Tetrahedral Polar Trigonal Planar Non-polar Trigonal pyramidal Polar Bent Polar Tetrahedral Non-polar BF3 NH3 H2O CH4 XeCl4 Square planar Non-polar Trigonal bipyramidal Non-polar Bent Polar Trigonal planar Non-polar Linear Non-polar Octahedral Non-polar Linear Non-polar Linear Non-polar PCl5 SO2 - CO32- - - N2 SF6 CO2 C O2 XeF5+ Square pyramidal Non-polar Post-lab Questions: 1. a) Draw four resonance structures for PO433- 3- - - - - 3- - - - 3- - - - b) Give the formal charge of each atom P= 0 3 single bonded oxygens = -1 double bonded oxygen = 0 c) Does having this resonance make phosphate more stable or less stable than an ion with no resonance structures? More stable, increase charge distribution.