VSEPR Theory Cheat Sheet: Molecular Geometry & Bond Angles
advertisement
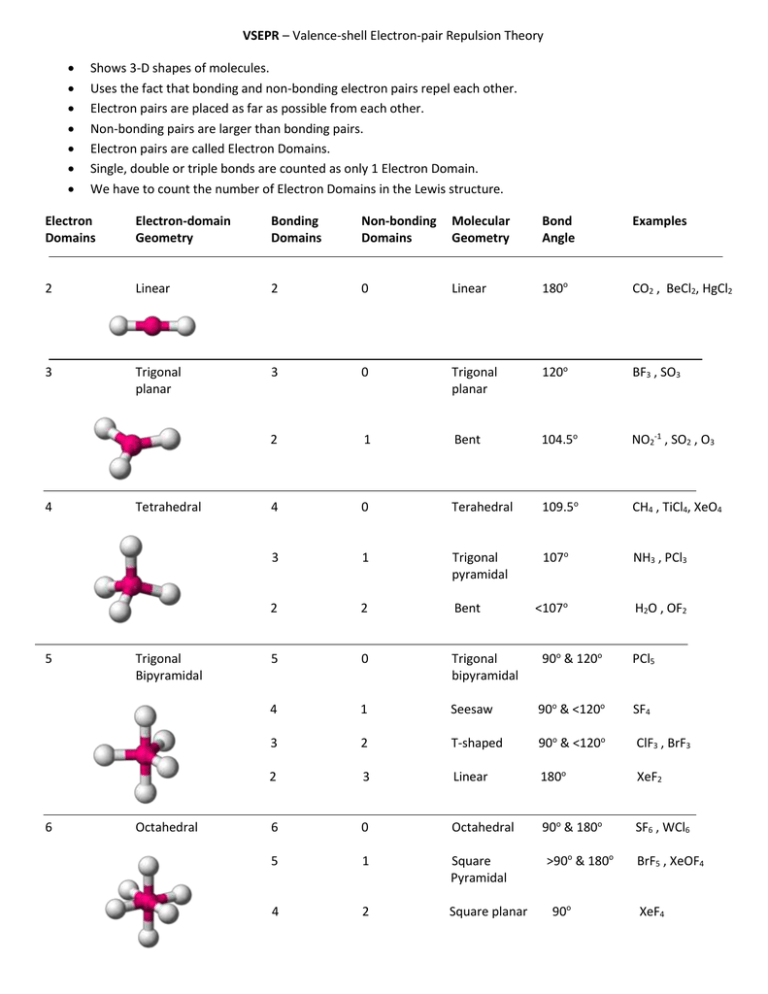
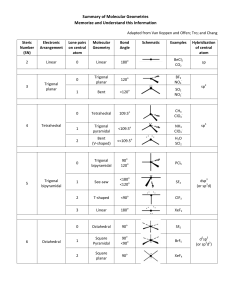
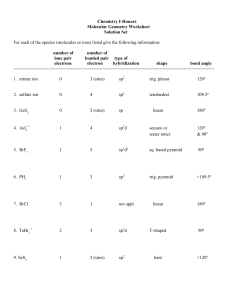
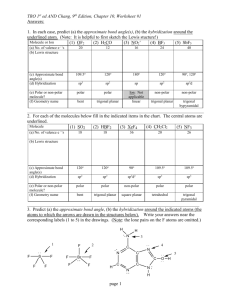
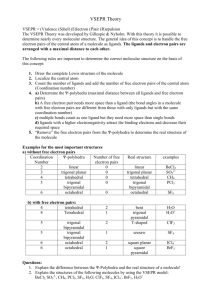
VSEPR – Valence-shell Electron-pair Repulsion Theory Shows 3-D shapes of molecules. Uses the fact that bonding and non-bonding electron pairs repel each other. Electron pairs are placed as far as possible from each other. Non-bonding pairs are larger than bonding pairs. Electron pairs are called Electron Domains. Single, double or triple bonds are counted as only 1 Electron Domain. We have to count the number of Electron Domains in the Lewis structure. Electron Domains Electron-domain Geometry Bonding Domains Non-bonding Domains Molecular Geometry Bond Angle Examples 2 Linear 2 0 Linear 180o CO2 , BeCl2, HgCl2 3 Trigonal planar 3 0 Trigonal planar 120o BF3 , SO3 2 1 Bent 104.5o NO2-1 , SO2 , O3 4 0 Terahedral 109.5o CH4 , TiCl4, XeO4 3 1 Trigonal pyramidal 107o NH3 , PCl3 2 2 Bent <107o H2O , OF2 5 0 Trigonal bipyramidal 90o & 120o PCl5 4 1 Seesaw 90o & <120o SF4 3 2 T-shaped 90o & <120o ClF3 , BrF3 2 3 Linear 180o XeF2 6 0 Octahedral 90o & 180o SF6 , WCl6 5 1 Square Pyramidal 4 2 Square planar 4 5 6 Tetrahedral Trigonal Bipyramidal Octahedral >90o & 180o 90o BrF5 , XeOF4 XeF4