VSEPR Exam Review
advertisement
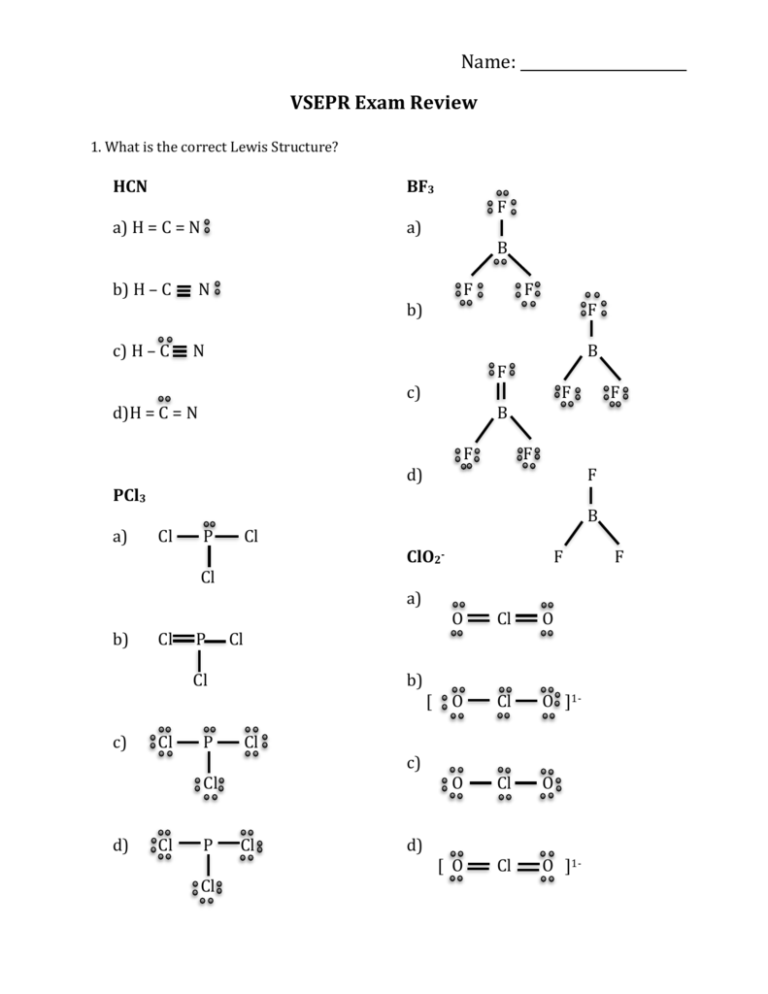
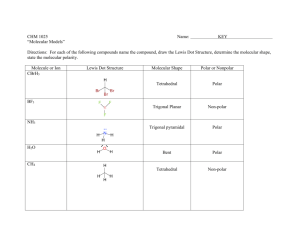
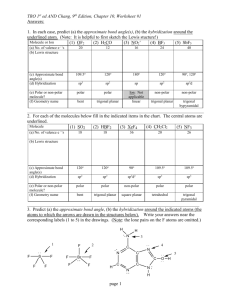
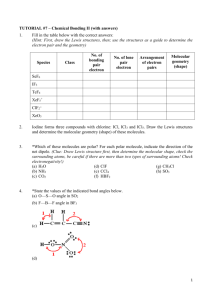
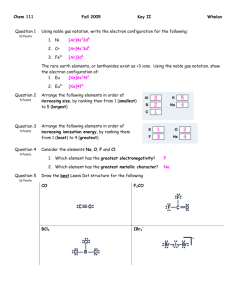
Name: VSEPR Exam Review 1. What is the correct Lewis Structure? HCN BF3 a) H = C = N a) b) H – C c) H – C N F B F B F d) F F F B Cl P Cl Cl P Cl P Cl b) Cl Cl Cl P Cl ClO2a) Cl d) F F c) Cl c) F N PCl3 b) B b) d)H = C = N a) F Cl c) d) [ F O Cl O O Cl O ]1- O Cl O [ O Cl O ]1- F 1) What is the molecular geometry for the following molecules? CCl4 a) See-saw b) Tetrahedral c) Square planar d) Linear BH3 a) T-Shaped b) Bent c) Trigonal Planar d) Trigonal Pyramidal SiS2 a) Bent c) Trigonal Planar d) T-Shaped b) Linear 2) Which of the following bonds is the shortest? a) Single bond b) double bond c) triple bond 3) ClF 3 has "T-shaped" geometry. There are a) 0 b) 1 c) 2 d) 3 non-bonding domains in this molecule. e) 4 4) The bond angles in a trigonal planar molecule are a) 120 b) 109.5 c) 90 d) 45 degrees. e) < 45 5) In the compound PF3 how many total valence electrons are present? a) 12 b) 21 c) 26 d) 6 6) How many electrons are there in the polyatomic ion, SO42-? a) 30 b) 31 c) 32 d) 28 7) The molecular geometry of the BCl3 molecule is _______, and this molecule is _______. a) trigonal pyramidal, polar d) trigonal planar, nonpolar b) trigonal pyramidal, nonpolar e) trigonal bipyramidal, polar c) trigonal planar, polar 8) NBr5 has __________ electron domains and a __________ molecular geometry. a) 6, trigonal bipyramidal d) 5, trigonal bipyramidal b) 6, tetrahedral e) 6, seesaw c) 5, square pyramidal 9) What is the electronegativity of fluorine? a) 1.0 b) 2.1 c) 3.5 d) 4.0 10) There are ___ bonding and ___ non-bonding pairs of electrons in a square planar molecule. a) 3, 2 b) 4, 2 c) 2, 2 d) 5, 1 For the following compounds: i) ii) iii) iv) v) Draw the Lewis Dot Structures for the following molecules (Include # of resonance structures if needed) Indicate the epg Indicate the mg Label the individual polar bonds with arrows Circle whether the entire compound is polar or non-polar a) SiH4 f) AsF3 epg: mg: polar or non-polar epg: mg: polar or non-polar b) H2S g) BCl3 epg: mg: polar or non-polar epg: mg: polar or non-polar c) NO21- h) KrCl4 epg: mg: polar or non-polar epg: mg: polar or non-polar d) SO32- i) BrI5 epg: mg: polar or non-polar epg: mg: polar or non-polar e) NH41+ j) SeCl6 epg: mg: polar or non-polar epg: mg: polar or non-polar **Calculate the formal charge for e) and j) above.