Sample Exam Questions midterm 3
advertisement

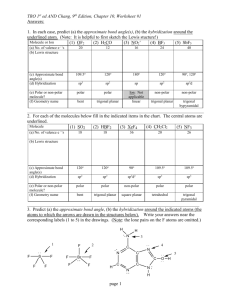
As citizens of earth we know we have to be careful about releasing greenhouse gasses into the environment. Define what a greenhouse gas is in molecular terms. Give me one example and show how your definition relates. It is also general knowledge that methane (CH4) is a greenhouse gas. Does this fit your definition above? Why or why not. A molecule with a dipole or an induced dipole. Yes, although CH bonds are not generally considered polar, there is a difference in electronegativity and by stretching in certain directions the bonds can be made so the vector addition of the CH bonds creates a dipole. Many correct examples. A) Draw the line structure of CH3CH(OH)CHCH2. What is the hybridization of the underline atoms. O: sp3 C:sp3 What is the hybridization of the atoms below for each bond tell me which orbitals are used for bonding. . (On the exam I would mark specific ones since obviously it would be too much to ask you to do all of them. Here just do all of them). Example answer for one of them. The Nitrogen in the ring: sp3 hybridized (note don’t forget about the lone pair that isn’t drawn in line structures). The bond to the hydrogen is a sigma bond formed with the nitrogen’s sp3 orbital and Hydrogen’s s orbital. The bond to the carbons (they happen to be identical in this case). Are formed with nitrogen’s sp3 orbital and carbons sp2 orbital. For each formula, draw the lewis structure, give the hybridization and geometry of the bolded element and state whether the molecule is polar. (Since this exam is mainly focused on the hybridization and polarity that is most likely what would be asked, however being able to do everything is good practice for the final and you are more likely to get the polarity correct if you know the proper geometries). A) PCl3 B) O3 C) BrF5 Hybridization __sp3 Geometry_____tetrahedral____ Is the molecule polar? ____yep Hybridization _sp3d2_ Geometry_______square pyramidal Is the molecule polar? _you betcha_ Hybridization ___sp2__ Geometry______bent_____ Is the molecule polar? ___yes_ Container A holds 762 mL of an ideal gas A at 2.30 atm. Container B holds 179 mL ideal gas B at 4.40 atm. If the gases are allowed to mix together, what is the resulting partial pressure of each gas and the total pressure? 1.86 atm, 0.836 atm, 2.70 atm At 1 atm and 0 oC, a 5.04 L mixture of methane (CH4) and propane (C3H8) was burned producing 20.9 g of CO2. How many moles total of methane and propane were present before combustion? How many moles of carbon dioxide were present after the reaction? What was the mole fraction of each gas in the mixture? Assume complete combustion. Total Moles before 0.225 mol moles CO2 after 0.457 mol, mol fraction CH4 0.444, mole C3H8 0.556 A 0.564 gram sample of a metal (M) reacts completely with sulfuric acid according to the reaction below. A volume of 219 mL of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside level. Atmospheric pressure is 756.0 Torr and the temperature is 25 oC. Calculate the molar mass of the metal. The vapor pressure of water at 25oC is 23.8 torr 65.4g.mol Note: All the book questions for this chapter are great. For extra practice for the gas law problems just do a lot of the book homework problems. This is just a sampling of ones I’ve happened to ask on old finals, but there are many many many different ways to ask gas law problems. No need to replicate them all here!