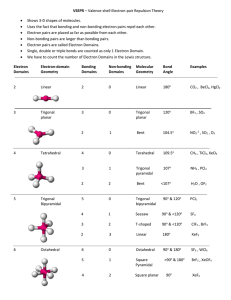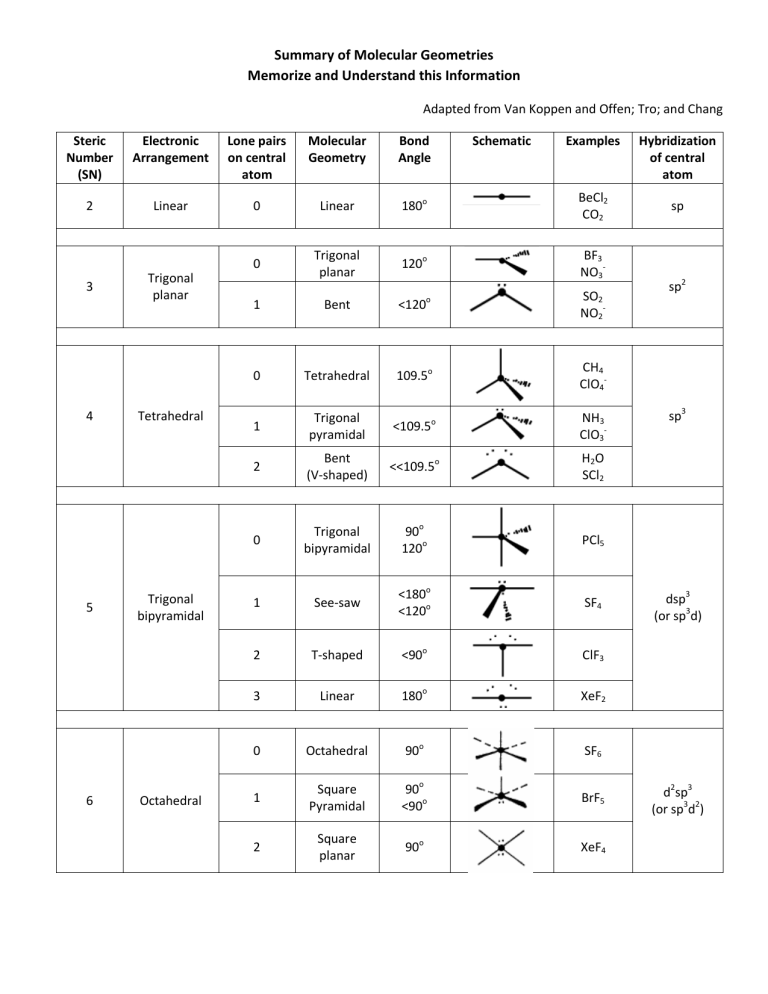
Summary of Molecular Geometries Memorize and Understand this Information Adapted from Van Koppen and Offen; Tro; and Chang Steric Number (SN) Electronic Arrangement Lone pairs on central atom Molecular Geometry Bond Angle 2 Linear 0 Linear 180o BeCl2 CO2 0 Trigonal planar 120o BF3 NO3- 3 4 5 6 Trigonal planar Tetrahedral Trigonal bipyramidal Octahedral Schematic Examples 1 Bent <120o SO2 NO2- 0 Tetrahedral 109.5o CH4 ClO4- 1 Trigonal pyramidal <109.5o NH3 ClO3- 2 Bent (V-shaped) <<109.5o H 2O SCl2 0 Trigonal bipyramidal 90o 120o PCl5 1 See-saw <180o <120o SF4 2 T-shaped <90o ClF3 3 Linear 180o XeF2 0 Octahedral 90o SF6 1 Square Pyramidal 90o <90o BrF5 2 Square planar 90o XeF4 Hybridization of central atom sp sp2 sp3 dsp3 (or sp3d) d2sp3 (or sp3d2)