Chapter 8 homework answers (Zumdahl andZumdahl) 7. done in
advertisement

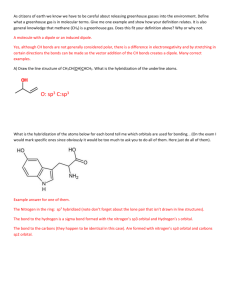
Chapter 8 homework answers (Zumdahl andZumdahl) 7. done in class 10. A chemical bond is a force of attraction holding 2 atoms together. Atoms form bonds with each other to lower their potential energy. This is due to the fact that most elements are higher in energy (have unpaired e- or unfilled orbitals) than bonded atoms. 24. True statements: a and c 26. True statement: c only a) XeF2 is linear and has 180 degree angle b) there are different bond angles d) they adopt a geometry to minimize repulsion 28. a) Rb, K, Na b) Ga, B, O c) Br, Cl, F d) S, O, F 80, 82, 88, 89 see scanned page 92. all bonds are a hybrid of single and double bonds (see structures on scanned page) 110. a) square pyramid (90 degree angles) b) square planar ( 90 degree angles c) octahedron ( 90 degree angles) 114. Which molecules are polar? 116 a) polar b) polar a only c) linear and nonpolar d) polar e) nonpolar f) polar 138. a) only CCl4 is nonpolar b) CO2 is nonpolar c) both are polar Chapter 9 answers 16. resonance structures show 2 single and 1 double bond. The true molecules is a hybrid of the 3 resonance forms so all bonds are equivalent. 24. All molecules in #82 have sp3 hybridization. In #88, sp2 hybridization. 26. All have sp3d2 hybridization. 38 a) see scanned page b) piperine has 0 sp, 11 sp2, 6 sp3 capsaicin has 0 sp, 9 sp2, 9 sp3 c) the nitrogens are sp3 hybridized in each molecule 60. a) see scanned page b) sulfurs have sp3 hybridization C’s with triple bonds: sp Most other C’s use sp2, the C in CH3 uses sp3 66. F2ClO2- is see saw (sp3d) F4ClO- is square pyramid (sp3d2) F2ClO+ is trigonal pyramid (sp3) F2ClO2+ is tetrahedron (sp3)