Continental Drift
advertisement
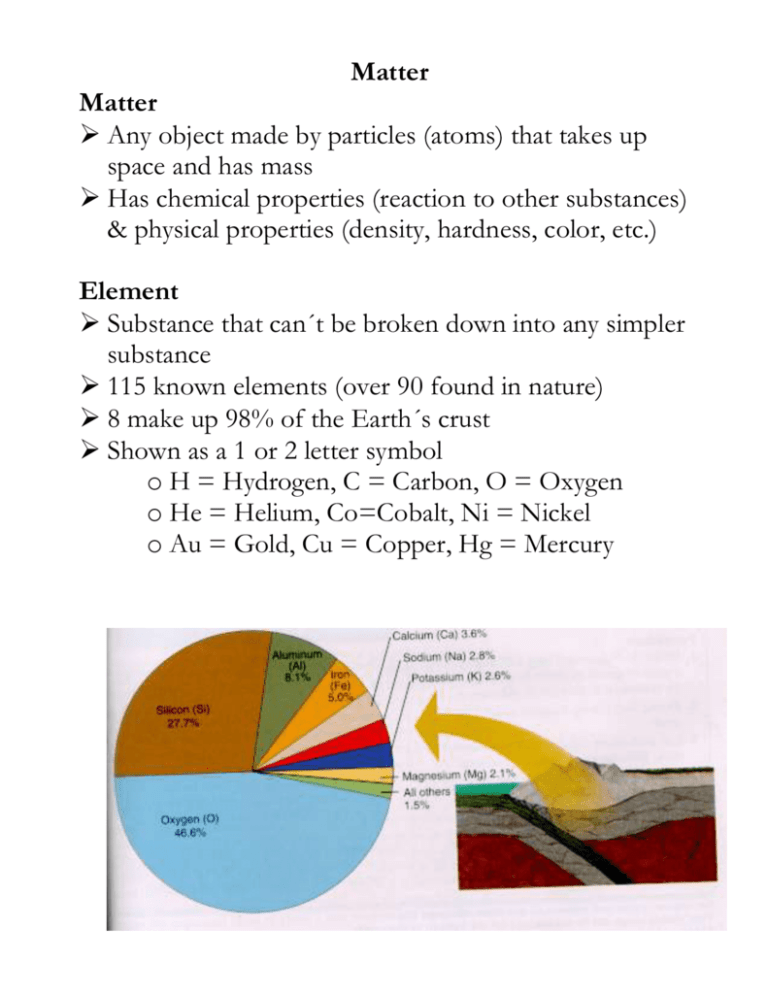
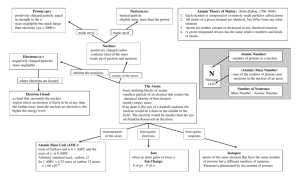
Matter Matter Any object made by particles (atoms) that takes up space and has mass Has chemical properties (reaction to other substances) & physical properties (density, hardness, color, etc.) Element Substance that can´t be broken down into any simpler substance 115 known elements (over 90 found in nature) 8 make up 98% of the Earth´s crust Shown as a 1 or 2 letter symbol o H = Hydrogen, C = Carbon, O = Oxygen o He = Helium, Co=Cobalt, Ni = Nickel o Au = Gold, Cu = Copper, Hg = Mercury The Atom Basic unit of all matter Made of 3 particles in 2 areas Subatomic Particles Proton o Positive electrical charge and a mass of 1 amu Electron o Negative electrical charge and no mass Neutron o No electrical charge (neutral) and mass of 1 amu Inside the Atom Atom is held together in part due to electrical attraction Like charges (+ and + or – and –) repel while unlike charges (+ and –) attract Atoms have equal numbers of protons ( + ) & electrons ( – ) so the overall charge is zero (neutral) Nucleus o Center core containing protons and neutrons o Has an overall positive charge Electron cloud o Area around nucleus which holds electrons in different energy levels o Has an overall negative charge Characterizing Atoms Atomic Number = number of protons o Determines the element you have Mass Number (Atomic Mass Number) = number of protons and neutrons Atomic Mass = total averaged mass of protons & neutrons in all types of an atom