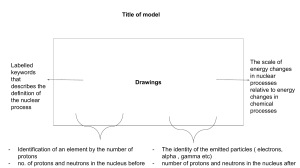
Lesson Plan Standard
advertisement

Lesson Plan Standard 7b: Students know each element has a specific number of protons in the nucleus (the atomic number) and each isotope of the element has a different but specific number of neutrons in the nucleus. Concepts Students will be able to describe what differentiates atoms from one another including how isotopes differ from one another. Objective Students will construct atoms online Students will keep track of the number of protons, electrons and neutrons using a spreadsheet Students will use excel to construct of graph of their findings. Procedure See student handout Enter all data in the excel file and observe graph and averages that are calculated. Analysis 1. Which particle controls what element an atom is? Describe how you used the model to come up with your answer. 2. What do you get when you change the number of neutrons in the nucleus? 3. What controls the "weight" of an atom? Describe how you used the model to come up with your answer. 4. What effect does varying the number of neutrons in the atom have on its stability? 5. What do you notice from the graph about atoms with the same number of protons?