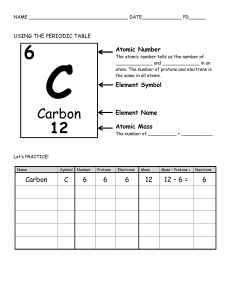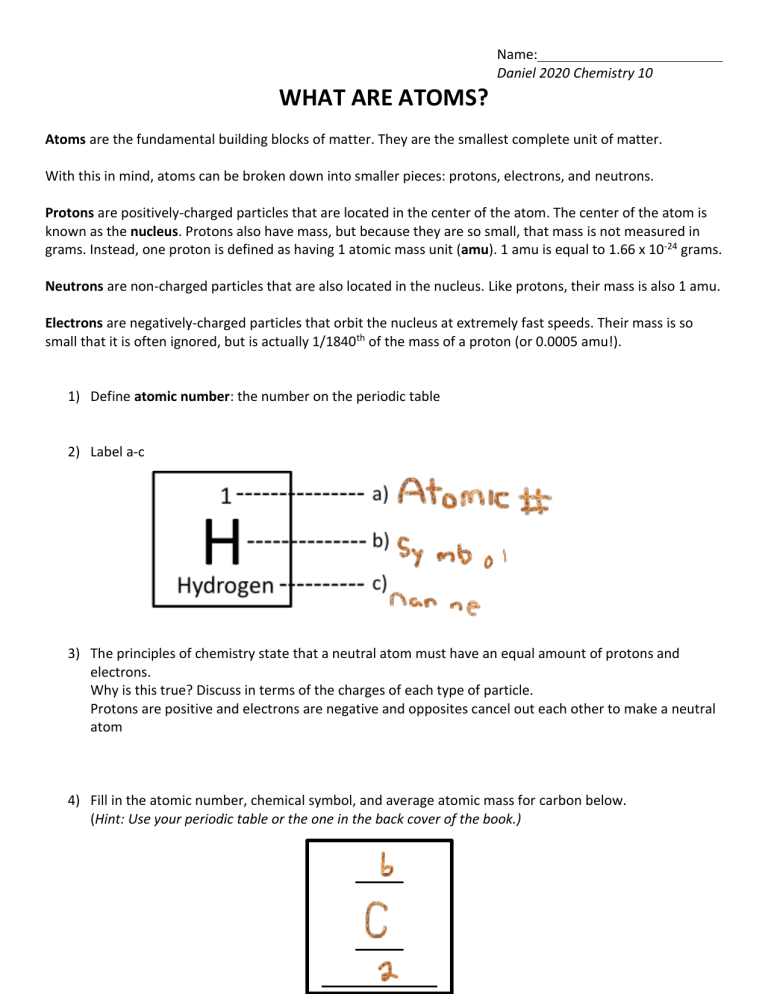
Name: Daniel 2020 Chemistry 10 WHAT ARE ATOMS? Atoms are the fundamental building blocks of matter. They are the smallest complete unit of matter. With this in mind, atoms can be broken down into smaller pieces: protons, electrons, and neutrons. Protons are positively-charged particles that are located in the center of the atom. The center of the atom is known as the nucleus. Protons also have mass, but because they are so small, that mass is not measured in grams. Instead, one proton is defined as having 1 atomic mass unit (amu). 1 amu is equal to 1.66 x 10-24 grams. Neutrons are non-charged particles that are also located in the nucleus. Like protons, their mass is also 1 amu. Electrons are negatively-charged particles that orbit the nucleus at extremely fast speeds. Their mass is so small that it is often ignored, but is actually 1/1840th of the mass of a proton (or 0.0005 amu!). 1) Define atomic number: the number on the periodic table 2) Label a-c 3) The principles of chemistry state that a neutral atom must have an equal amount of protons and electrons. Why is this true? Discuss in terms of the charges of each type of particle. Protons are positive and electrons are negative and opposites cancel out each other to make a neutral atom 4) Fill in the atomic number, chemical symbol, and average atomic mass for carbon below. (Hint: Use your periodic table or the one in the back cover of the book.)