The Atom- Review Which statement is consistent with the results of
advertisement

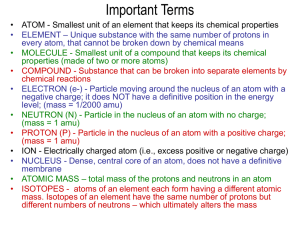
Name __________________________ Date___________________________ The Atom- Review 1. Which statement is consistent with the results of Rutherford’s gold foil experiment? a. b. c. d. All atoms have a positive charge Atoms are mostly empty space The nucleus of an atom contains protons and electrons Mass is spread uniformly throughout an atom 2. How many protons are found in an atom of each of the following? a. Boron b. Sulfur c. Neon d. Lithium 3. How did the results of Rutherford’s gold foil experiment differ from his expectations? 4. How many protons are in the nuclei of the following atoms? a. Fluorine b. Argon c. Manganese 5. How many neutrons are in the nuclei of the following atoms? a. Scandium b. Rubidium c. Nickel 6. What is the difference between the mass number and the atomic number of an atom? 7. Complete the table for the following elements: Element Manganese Sodium Bromine Yttrium Arsenic Actinium Symbol Number of Protons 25 Number of Electrons 11 35 Number of neutrons 30 12 45 33 8. Name two ways that isotopes of an element differ. Atomic Number Mass Number 39 89 75 227 9. How many neutrons are in each atom? a. 23 b. 238 c. 81 d. 19 11 92 35 9 Na U Br F ______________ ______________ _______________ _______________ 10. The two most abundant isotopes of carbon are carbon-12 (mass = 12.00 amu) and carbon-13 (mass= 13.00 amu). Their relative abundances are 98.9 % and 1.10% respectively. Calculate the atomic mass of carbon.